EASY
Earn 100
The heat required to raise the temperature of a body by 1 K is called
(a)Specific heat
(b)Thermal capacity
(c)Water equivalent
(d)None of these
50% studentsanswered this correctly
Important Questions on Chemical Thermodynamics
EASY
MEDIUM
(R = 8.314 J/mol K) (ln7.5 = 2.01)
MEDIUM
EASY
EASY
HARD

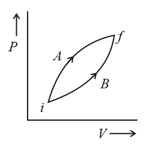
The above diagram represents the thermodynamic cycle of an engine, operating with an ideal mono-atomic gas. The amount of heat, extracted from the source in a single cycle, is:
MEDIUM
MEDIUM
EASY
EASY
EASY
EASY
EASY
EASY
MEDIUM
EASY
EASY
EASY
EASY
i)
ii)
iii)
iv)

