EASY
Earn 100
The hybridisation states of carbon atom bonded to halogen atom in haloalkanes and haloarenes are and respectively.
(a)True
(b)False
50% studentsanswered this correctly
Important Questions on Haloalkanes and Haloarenes
HARD
The CORRECT match of compound in Column with density in Column is
| Column A | Column B | ||
| p | i. | ||
| q | ii. | ||
| r | iii. | ||
| s | iv. |
MEDIUM
EASY
Classify the following compounds into haloalkanes and haloarenes.
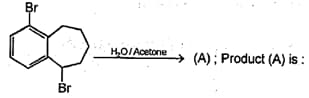
MEDIUM
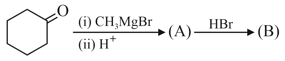
in the above reaction is
EASY
MEDIUM
EASY
MEDIUM
The correct order of rate of reaction is:
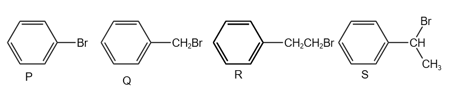
MEDIUM
MEDIUM
MEDIUM
EASY
MEDIUM
EASY
MEDIUM
EASY
MEDIUM
MEDIUM
EASY