EASY
Earn 100
The incorrect statement for kinetic molecular theory of gases is
(a)Particles of a gas are always in random motion
(b)There exists forces of attraction between the particles of a gas at ordinary temperature and pressure
(c)Collision of gas molecules are perfectly elastic
(d)Different particles in the gas may have different speeds
50% studentsanswered this correctly
Important Questions on States of Matter: Gaseous and Liquid States
EASY
Predict the correct order of rate of diffusion of the following molecules.
MEDIUM
Identify the correct labels of and in the following graph from the options given below:
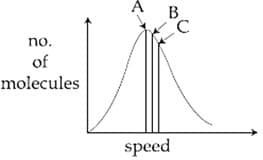
Root mean square speed most proable speed Average speed
EASY
The internal energy of one mole of an ideal gas is given by the expression:
MEDIUM
What is the kinetic energy (in ) of the nitrogen molecule at ?
EASY
At room temperature, the average speed of Helium is higher than that of Oxygen by a factor of:
MEDIUM
At what temperature in, the most probable velocity of ozone gas is equal to RMS velocity of oxygen gas at
HARD
If the distribution of molecular speeds of a gas is as per the figure shown below, then the ratio of the most probable, the average, and the root mean square speeds, respectively, is
MEDIUM
Among the following, the plot that shows the correct marking of most probable velocity average velocity and root mean square velocity is :
MEDIUM
The temperature at which oxygen molecules have the same root mean square speed as helium atoms have at 300 K is : (Atomic masses : He = 4 u, O = 16 u)
EASY
Initially, the root-mean-square (RMS) velocity of molecules at certain temperature is . If this temperature is doubled and all the nitrogen molecules dissociate into nitrogen atoms, then the new RMS velocity will be:
MEDIUM
If the kinetic energy and speed of a gas at a certain temperature are and , respectively. The molecular weight of the gas is
MEDIUM
Which of the following is not an assumption of the kinetic theory of gases?
EASY
The quantity corresponds to
MEDIUM
The kinetic energy in of mole of at is:
EASY
If the r.m.s. speed of a gaseous molecule is at a pressure , then what will be the r.m.s. speed at a pressure and constant temperature ?
EASY
What will be the relation between the of gas with and of gas with if the average speed of gas is equal to most probable speed of gas
EASY
The rms velocity of hydrogen is times the velocity of nitrogen. If is the temperature of the gas, then
MEDIUM
Points and in the following plot respectively correspond to ( most probable velocity)
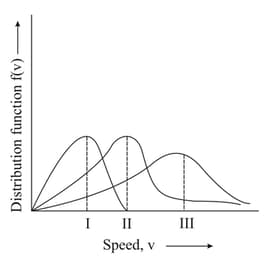
EASY
The ratio of root-mean-square velocity of hydrogen at to that of nitrogen at is closest to
EASY
For gaseous state, if most probable speed is denoted by , average speed by and root mean square speed by , then for many molecules, what is the ratios of these speeds?

