MEDIUM
11th CBSE
IMPORTANT
Earn 100
The intermediate carbocation formed in the reactions of and with propene is the same and the bond energy of and is and respectively. What will be the order of reactivity of these halogen acids?

Important Questions on Hydrocarbons
HARD
11th CBSE
IMPORTANT
What will be the product obtained as a result of the following reaction and why?
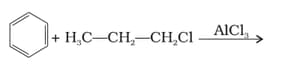
MEDIUM
11th CBSE
IMPORTANT
MEDIUM
11th CBSE
IMPORTANT
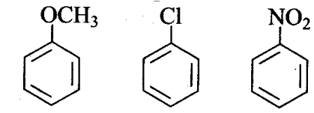
Arrange the following set of compounds In the order of their decreasing relative reactivity with an electrophile. Give reason.
MEDIUM
11th CBSE
IMPORTANT
MEDIUM
11th CBSE
IMPORTANT
MEDIUM
11th CBSE
IMPORTANT
HARD
11th CBSE
IMPORTANT
Predict the major product (s) of the following reactions and explain their formation.
MEDIUM
11th CBSE
IMPORTANT
Nucleophiles and electrophiles are reaction intermediates having electron-rich and electron-deficient centres respectively. Hence, they tend to attack electron-deficient and electron-rich centres respectively. Classify the following species as electrophiles and nucleophiles.