EASY
Earn 100
The intermolecular attraction that is dependent on the inverse cube of the distance between the molecules is
(a)Ion-ion interaction
(b)Ion-dipole interaction
(c)London force
(d)Hydrogen bond
50% studentsanswered this correctly
Important Questions on States of Matter
MEDIUM
In which of the following solid substance dispersion forces exist?
MEDIUM
EASY
[ is the distance between the polar molecules]
HARD
EASY
EASY
Three types of potential energy due to attractive interaction between two species and are represented by the curves and in the figure below.
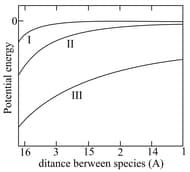
Consider the dominating interaction between non-rotating species for , and . The correct assignment of the ... interactions
to the types , and is:
EASY
EASY
Increasing order of boiling points in the following compounds is:
EASY
MEDIUM
Match the type of interaction in column with the distance dependence of their interaction energy in column
| A | B |
| (i) ion - ion | (a) |
| (ii) Dipole - dipole | (b) |
| (iii) London dispersion | (c) |
| (d) |
EASY
EASY
EASY
EASY
EASY
EASY
EASY
EASY
EASY
(i) n-hexane and n-octane
(ii) I2 and CCl4
(iii) NaClO4 and water
(iv) Methanol and acetone
(v) Acetonitrile (CH3CN) and acetone (C3H6O)
Make selections from the following choices.
HARD

