The lattice energy of magnesium bromide, can be calculated using the enthalpy changes shown in the table.
Type of enthalpy change
Value /
first ionisation energy of magnesium
second ionisation energy of magnesium
first electron affinity of bromine
enthalpy change of formation of
enthalpy change of atomisation of magnesium
enthalpy change of atomisation of bromine
Draw and label a Born-Haber cycle to calculate the lattice energy of magnesium bromide and calculate the lattice energy of magnesium bromide.

Important Questions on Lattice Energy
In some crystal lattices, some of the ions are polarised. State the meaning of the term ion polarisation.
The diagram shows the enthalpy changes when sodium chloride is dissolved in water.
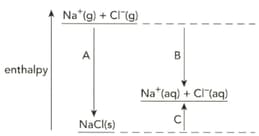
Define the term enthalpy change of solution.
The diagram shows the enthalpy changes when sodium chloride is dissolved in water.
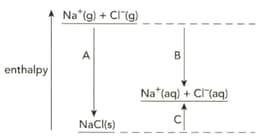
Write symbol equation that describe the enthalpy change of solution of sodium chloride.
The diagram shows the enthalpy changes when sodium chloride is dissolved in water.
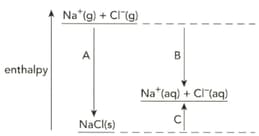
Name the enthalpy changes labelled A, B and C.
