The main product formed by the reaction of methane amine with Tilden reagent is-

Important Points to Remember in Chapter 13 - Amines from Embibe Experts Achieve CUET (UG) Chemistry Practice Book Solutions
Amines can be considered as derivatives of ammonia, obtained by replacement of one, two or all the three hydrogen atoms by alkyl and/or aryl groups.
2. Preparation of Amines:
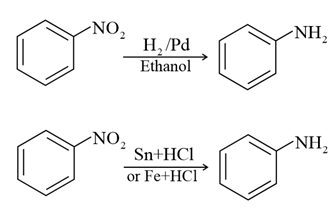
(i) By reduction of nitro compounds-
(ii) By Ammonolysis of alkyl halides :
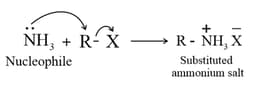
(iii) The free amine can be obtained from the ammonium salt by treatment with a strong base.
(iv) Reduction of nitriles
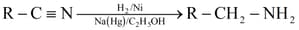
(v) Reduction of amides
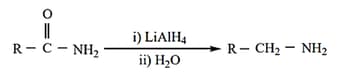
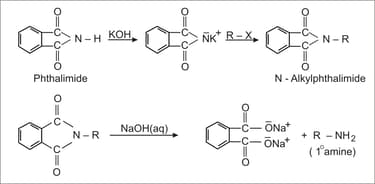
(vi) Gabriel synthesis is used for the preparation of primary amines. Phthalimide on treatment with ethanolic potassium hydroxide forms potassium salt of phthalimide which on heating with alkyl halide followed by alkaline hydrolysis produces the corresponding primary amine.
(vii) Hoffmann bromamide reaction :
3. Physical and chemical properties of amines:
(i) The order of boiling points of isomeric amines is primary > secondary > tertiary.
(ii) Because of the presence of a lone pair of electrons on the nitrogen atom of group, amines behave as Lewis bases.
(iii) All aliphatic amines are more basic than ammonia. In aqueous solution, the order of basic character is
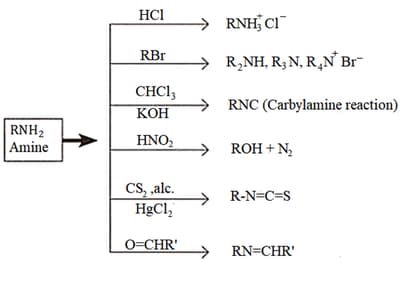
4. Reactions of Amines:
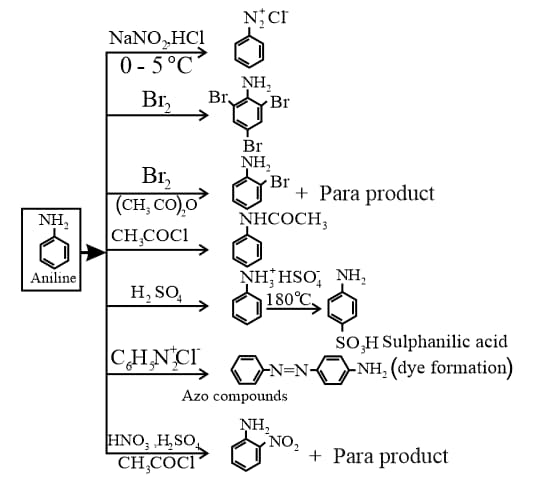
5. Reactions of Aniline:
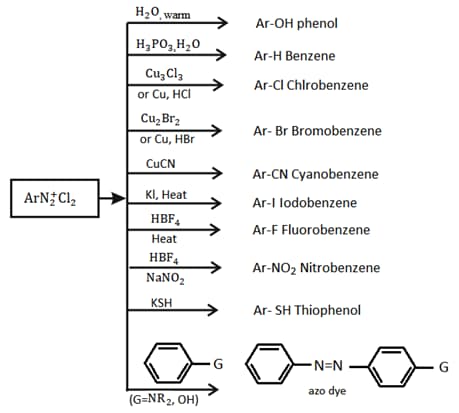
6. Properties of Diazonium Salts: