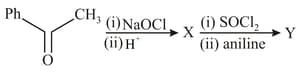The mass of produced when of cyclohexane carbaldehyde undergoes Tollen’s test is _____ . (Nearest Integer)
Molar mass of
Important Questions on Aldehydes and Ketones
Given below are two statements :
Statement : The nucleophilic addition of sodium hydrogen sulphite to an aldehyde or a ketone involves proton transfer to form a stable ion.
Statement : The nucleophilic addition of hydrogen cyanide to an aldehyde or a ketone yields amine as final product.
In the light of the above statements, choose the most appropriate answer from the options given below :
Which is the most suitable reagent for the following conversion?
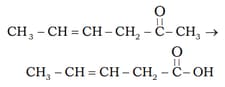
Identify the product of the following reaction :
Which of the following reagents are used to distinguish aldehydes from Ketones?
I. Fehling's reagent
II. Lucas reagent
III. Hinsberg's reagent
IV. Tollens reagent
Give the plausible explanation for the following:
Glucose doesn’t give DNP test.
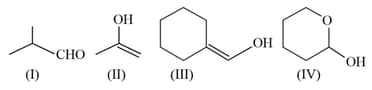
Which among the above compound/s does/do not form Silver mirror when treated with Tollen's reagent?
The major products of the following reaction.
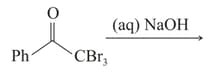 are
are