The mechanical equivalent of heat

Important Points to Remember in Chapter -1 - Calorimetry from H C Verma CONCEPTS OF PHYSICS [VOLUME 2] Solutions
1. Concept of temperature:
(i) Temperature is the degree of hotness or coldness of a body. Flow of heat is from higher temperature to lower temperature.
(ii) When two bodies are at the same temperature no heat flows from one body to the other (Thermal Equilibrium).
(iii) Temperature is a fundamental scalar physical quantity. S.I. unit kelvin. Dimension =
(iv) or implies or
2. Temperature scales:
| Name of the scale | Symbol for each degree | Lower fixed point (LFP) | Upper fixed point (UFP) | Number of divisions on the scale |
| Celsius | ||||
| Fahrenheit | ||||
| Kelvin |

3. Thermometry:
In some substances, few physical properties vary linearly with the change of temperature and it is used for temperature measurement.
(i) Length of liquid in the tube
(ii) Pressure of gas (at constant volume).
(ii) Volume of gas (at constant pressure).
4. Types of thermometers:
(i) Mercury thermometers: Mercury has large thermal coefficient of expansion and change is uniform. It also has high thermal conductivity and low specific heat, two desirable properties for good thermometers.
(ii) Constant pressure gas thermometers
If pressure is constant, then .
(iii) Constant volume gas thermometers
If volume is constant, then
5. Thermal expansion:
Increase in temperature results in an increase in the amplitude of vibration and energy of atoms which leads to
increase of distance between the atoms.
(i) Linear expansion (In solids )
(a)
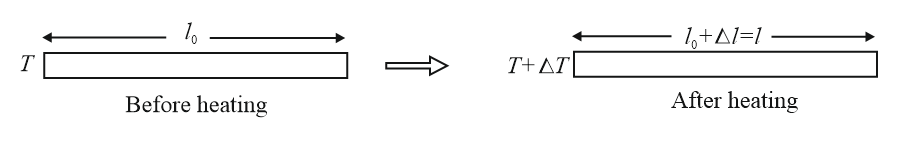
(b) Unit of : or
(ii) Areal expansion (In solids )
(a)
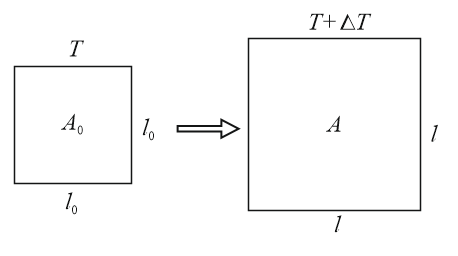
(b) Unit of :
(iii) Volume expansion (In solids, liquids and gases )
(a)
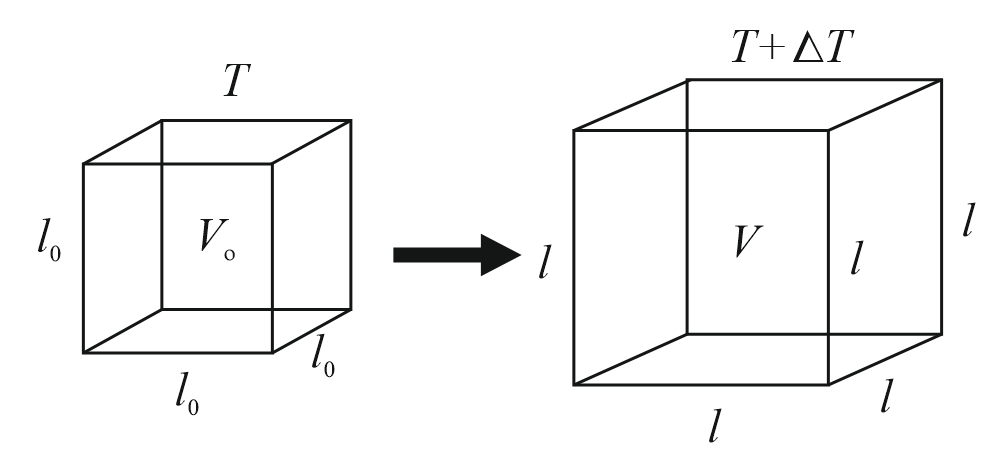
(b) Unit of :
(iv) For isotropic solids
(v) Thermal expansion of an isotropic object may be imagined as a photographic enlargement.
(vi) For anisotropic materials and
(vii) If is variable:
6. Thermal expansion applications
(i) Bi-metallic strip: Different materials have different coefficient of linear expansion and if joined together the strip will bend with metal of greater on outer side(convex side). It is used to make or break electrical contact.
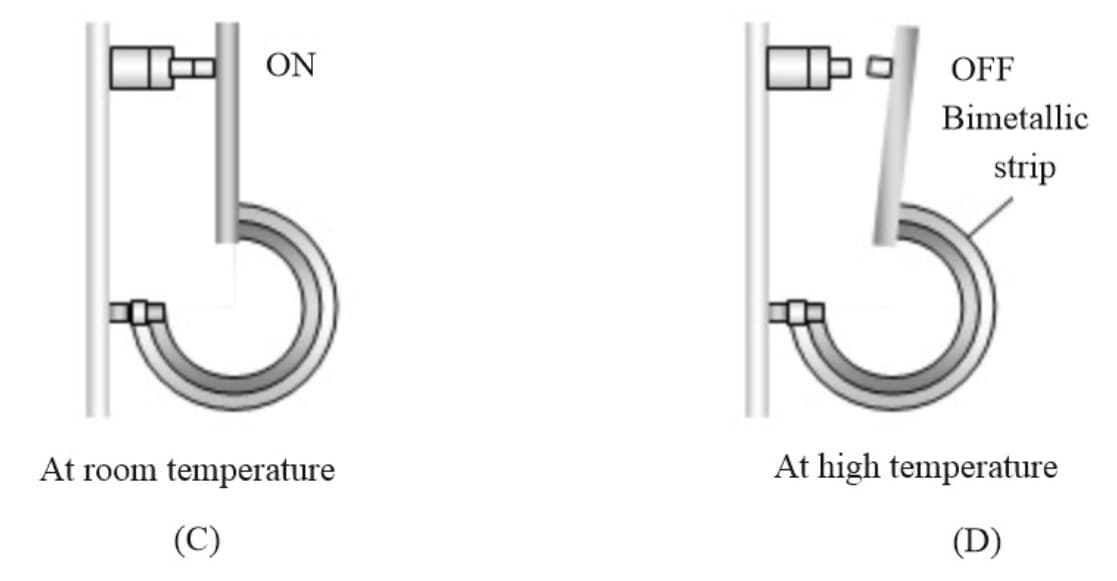
(ii) Effect on the time period of a simple pendulum:
As the temperature is increased length of the pendulum increases due to change thermal expansion & hence time period will increase. It becomes slower in summer & loses time.
Fractional change in time-period
(iii) Thermal stress: If rod ends are fixed to prevent expansion or contraction then due to thermal expansion or contraction, compressive or tensile stress is developed. Hence rod will apply a large force on the wall.
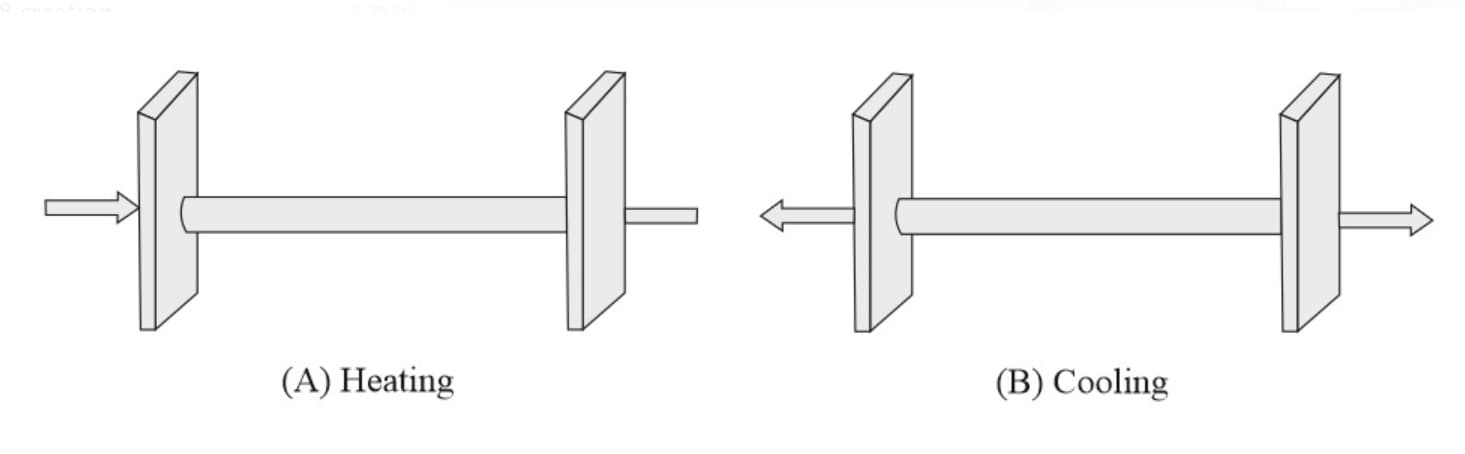
(a) Thermal strain
(b) Thermal stress
(c) Force developed
(iv) Error in scale reading:
If scale gives correct reading at temperature, then at temperature it will expand and the reading will be lesser than the true value.
True value = Scale reading
7. Thermal expansion of gases:
(i) Coefficient of volume expansion
at constant pressure
(ii) Coefficient of pressure expansion
8. Thermal expansion in liquids:
On heating both liquid & vessel expands therefore, increase in the volume of the liquid = The apparent increase in the volume of liquid + the increase in the volume of the vessel.
(i) Co-efficient of apparent expansion : It is due to apparent (that appears to be, but is not) increase in the volume of liquid if expansion of vessel containing the liquid is not taken into account.
(ii) Co-efficient of real expansion : It is due to the actual increase in volume of liquid due to heating.
(iii)
(iv) Anomalous expansion of water:
It expands on heating if its temperature is greater than . In the range to , water contracts on heating and expands on cooling, i.e., is negative. This behaviour of water in the range from to is called anomalous expansion.
9. Heat:
A form of energy that is transferred between objects with different temperatures. It is a scalar quantity.
Unit:
(i) Thermal capacity of a body
It is the amount of heat required to raise the temp. of a given body by or .
(iii) Specific heat capacity (mass)
It is the amount of heat required to raise the temperature of unit mass of a body through or .
(iv) Molar heat
(v) Water equivalent: Mass of water which when given the same amount of heat as to the body, changes.
the temperature of water through the same range as that of the body.
Water equivalent of the body,
Unit: or .
(vi) Latent Heat (Hidden heat): The amount of heat that has to be supplied to (or removed from) a body for
its complete change of state (from solid to liquid, liquid to gas etc.) is called latent heat of the body. Remember that phase transformation is an isothermal (i.e. temperature = constant) change.
10. Principle of calorimetry:
Heat lostheat gained.
For temperature change and for phase change
11. Heating curve:
If to a given mass of a solid, heat is supplied at a constant rate and a graph is
plotted between temperature and time, the graph is called the heating curve.
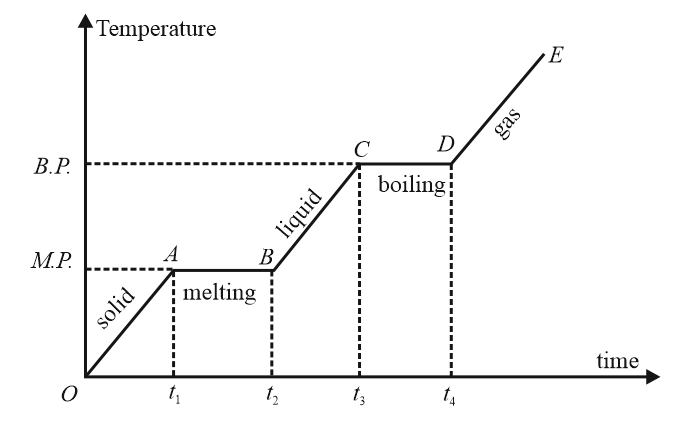
(i) Specific heat (or thermal capacity)
(ii) Latent heat length of horizontal line.
12. General points:
(i) Specific heat of a body may be greater than its thermal capacity as mass of the body may be less than unity.
(ii) The steam at causes more severe burn to human body than the water at because steam has
greater internal energy than water due to latent heat of vaporization.
(iii) Heat is energy in transit which is transferred from hot body to cold body.
(iv) One calorie is the amount of heat required to raise the temperature of one gram of water through
(more precisely from to ).
Clausius-Clapeyron equation (effect of pressure on the boiling point of liquids & melting point of solids related
with latent heat)
