EASY
10th Maharashtra Board
IMPORTANT
Earn 100
The molecular formula ethyne is . From this draw its structural formula and electron-dot structure.

Important Questions on Carbon Compounds
EASY
10th Maharashtra Board
IMPORTANT
MEDIUM
10th Maharashtra Board
IMPORTANT
EASY
10th Maharashtra Board
IMPORTANT
Fill in the gaps in the table of homologous series.
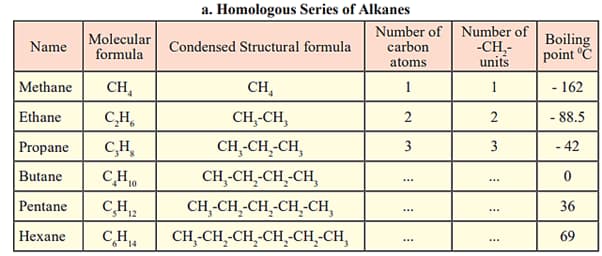
EASY
10th Maharashtra Board
IMPORTANT
Fill in the gaps in the table of homologous series.
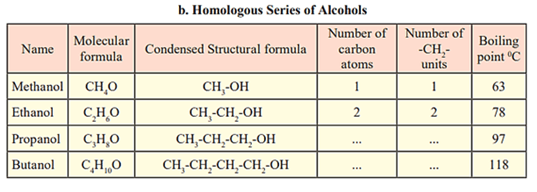
EASY
10th Maharashtra Board
IMPORTANT
Fill in the gaps in the table of homologous series.
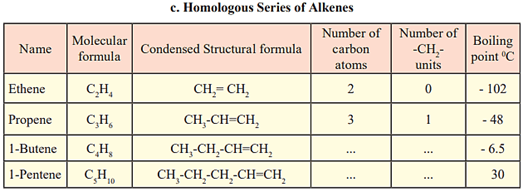
EASY
10th Maharashtra Board
IMPORTANT
EASY
10th Maharashtra Board
IMPORTANT
EASY
10th Maharashtra Board
IMPORTANT
