MEDIUM
10th Maharashtra Board
IMPORTANT
Earn 100
The molecular formula of sulphur is in which eight sulphur atoms are bonded to each other to form one ring. Draw an electron-dot structure for .

Important Questions on Carbon Compounds
EASY
10th Maharashtra Board
IMPORTANT
Hydrogen peroxide decomposes on its own by the following reaction.
From this, what will be your inference about the strength of covalent bond?
EASY
10th Maharashtra Board
IMPORTANT
Hydrogen peroxide decomposes on its own by the following reaction.
Tell from the above example whether oxygen has catenation power or not.
EASY
10th Maharashtra Board
IMPORTANT
EASY
10th Maharashtra Board
IMPORTANT
EASY
10th Maharashtra Board
IMPORTANT
MEDIUM
10th Maharashtra Board
IMPORTANT
EASY
10th Maharashtra Board
IMPORTANT
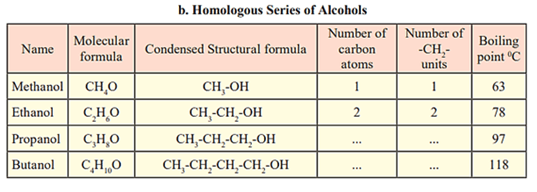
Fill in the gaps in the table of homologous series.
EASY
10th Maharashtra Board
IMPORTANT
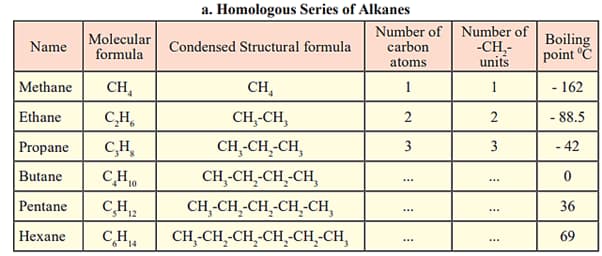
Fill in the gaps in the table of homologous series.