The number of incorrect statement/s from the following is ______________
A. The successive half lives of zero order reactions decreases with time.
B. A substance appearing as reactant in the chemical equation may not affect the rate of reaction
C. Order and molecularity of a chemical reaction can be a fractional number
D. The rate constant units of zero and second order reaction are and respectively
Important Questions on Chemical Kinetics
Which of the following relation is correct for zero-order reaction?
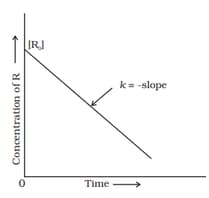
Examine the graph given below. Identify the integrated rate equation and the order of the reaction corresponding to it.
The given graph is a representation of kinetics of a reaction.
The and axes for zero and first order reactions, respectively are
Derive the integrated rate equation of Zero Order Reaction.