MEDIUM
Earn 100
The number of lone pairs of electrons on in a molecule is____.
50% studentsanswered this correctly
Important Questions on Elements of Groups 16, 17 and 18
MEDIUM
Two compounds and can react with to form cationic and anionic species respectively. and can be
MEDIUM
mole of is treated with of water. The product obtained is
MEDIUM
Which of the following is not formed during the hydrolysis (partial / full) of ?
EASY
on complete hydrolysis gives
MEDIUM
Xenon hexafluoride on partial hydrolysis produces compounds 'X' and 'Y' and the respective oxidation states of Xe are:
MEDIUM
The number of lone pair of electron and hybridisation in is
MEDIUM
Among the following molecules,
(i)
(ii)
(iii)
those having same number of lone pairs on are:
MEDIUM
Which of the following compound of Xenon possesses square pyramidal structure?
MEDIUM
Match the compounds given in column I with the hybridization and shape given in column II and mark the correct option.
| Column I | Column II | ||
| (a) | (i) | Distorted octahedral | |
| (b) | (ii) | Square planar | |
| (c) | (iii) | Pyramidal | |
| (d) | (iv) | Square pyramidal |
HARD
Under ambient conditions, the total number of gases released as products in the final step of the reaction scheme shown below is
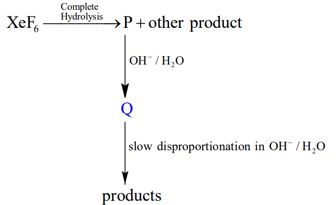
EASY
The complete hydrolysis of result in the formation of
EASY
on partial hydrolysis with water, produces a compound The same compound is formed when reacts with silica. The compound is:
MEDIUM
In equation, , containing product is
HARD
Match List-I with List-II and select the correct answer using following codes
| List-I | List-II | |
| A | Regular tetrahedron | |
| B | Distorted octahedron | |
| C | Linear | |
| D | Square planer | |
| E | Pyramidal |
EASY
reacts with to give
MEDIUM
is isostructural with
MEDIUM
Partial hydrolysis of gives
MEDIUM
Match the Xenon compounds in Column-I with its structure in Column-II and assign the correct code:
| Column-I | Column-II | ||
| pyramidal | |||
| square planar | |||
| distorted octahedral | |||
| square pyramidal | |||
EASY
on complete hydrolysis yields ''. The molecular formula of and its geometry, respectively, are:
EASY
In the number of lone pairs on are respectively

