The orbital frequency of an electron in the hydrogen atom is proportional to
Important Questions on Structure of Atom
For any given series of spectral lines of atomic hydrogen, let be the difference in maximum and minimum wave number in .
The ratio is
Given below are two statements :
Statement I : According to Bohr's model of an atom, qualitatively the magnitude of velocity of electron increases with decrease in positive charges on the nucleus as there is no strong hold on the electron by the nucleus.
Statement II : According to Bohr's model of an atom, qualitatively the magnitude of velocity of electron increases with decrease in principle quantum number.
In the light of the above statements, choose the most appropriate answer from the options given below:
| List - I | List - II |
|---|---|
| (I) Radius of the orbit | |
| (II) Angular momentum of the electron in the orbit | |
| (III) Kinetic energy of the electron in the orbit | |
| (IV) Potential energy of the electron in the orbit | |
Which of the following options has the correct combination considering List-I and List-II?
The longest wavelength of light that can be used for the ionisation of lithium atom in its ground state is . The value of is (Nearest Integer)
(Given : Energy of the electron in the first shell of the hydrogen atom is ; and )
As wavelength decreases, the lines in the series converge.
The integer is equal to .
The lines of the longest wavelength correspond to .
The ionization energy of hydrogen can be calculated from the wave number of these lines.
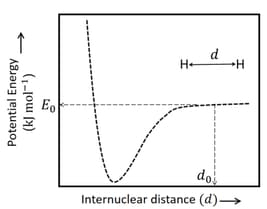
The figure below is the plot of potential energy versus internuclear distance of molecule in the electronic ground state. The value of the net potential energy (as indicated in the figure) is for at which the electron-electron repulsion and the nucleus-nucleus repulsion energies are absent, find the value of to the nearest integer value.
As reference, the potential energy of atom is taken as zero when its electron and the nucleus are infinitely far apart.
Use Avogadro constant as Give an answer to the nearest integer value.
| List - I | List - II |
|---|---|
| (I) Radius of the orbit | |
| (II) Angular momentum of the electron in the orbit | |
| (III) Kinetic energy of the electron in the orbit | |
| (IV) Potential energy of the electron in the orbit | |
Which of the following options has the correct combination considering List-I and List-II?
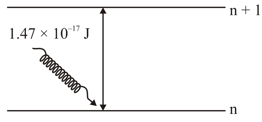
The electron in the orbit of is excited to orbit using the radiation of energy (as shown in the diagram). The value of is ___________
Given :
The ionization energy of gaseous atoms is . The lowest possible frequency of light that ionizes a sodium atom is

