The value of four halo phenols is given below:
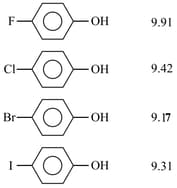
Consider the following statements regarding value:
(1) and atoms have both and effects
(2) In case of -chloro, -bromo, and -iodophenoxide ions, and acts as accept (- effect)
(3) Due to acceptor nature of halogen atom, the negative charge on oxygen of phenoxide ion gets delocalised and hence effect predominates.
(4) The accepting ability order is as . Hence -iodophenol is most acidic.
Of the these the correct statements are :

Important Questions on Alcohols, Phenols and Ethers
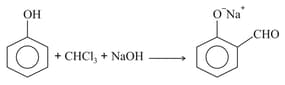
The functional group which is formed when Phenol is made to react with Chloroform in the presence of dilute Sodium hydroxide
The major product of the following reaction is:
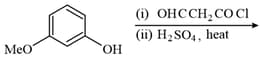
The major product of the following reaction is:
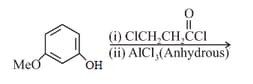
What is the product "C" after following reactions -
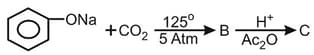
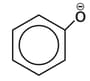 ion ?
ion ?| Test | Inference | |
|---|---|---|
| Insoluble | ||
| Soluble | ||
| Decolourization |
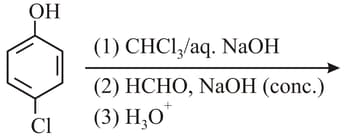
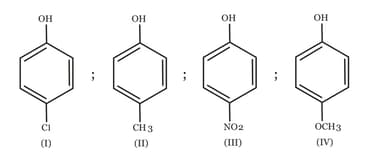
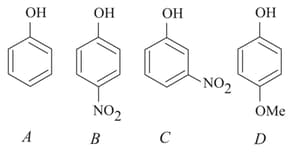
The increasing order of the values of the following compounds is:
What is in the following sequence of reactions?