The pattern in Hexagonal close packing and cubic close packing are same. Is the statement true or false?
Important Questions on The Solid State
A two dimensional solid is made by alternating circles with radius and such that the sides of the circles touch. The packing fraction is defined as the ratio of the area under the circles to the area under the rectangle with sides of length and
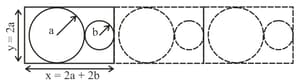
The ratio for which the packing fraction is minimized is closest to:
What are the examples of hexagonal close-packed structure?
The number of atoms in the hcp unit cell is_____.
A metallic element crystallises into a lattice containing a sequence of layers Any packing of spheres leaves out voids in the lattice. What percentage by volume of this lattice is empty space?
Represent the union of two sets by Venn diagram for each of the following.
is a prime number between and
is an odd number between and

