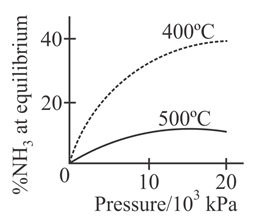
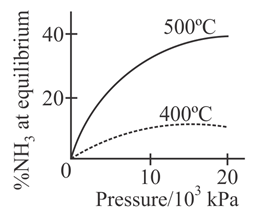
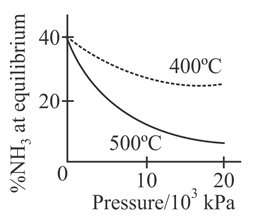
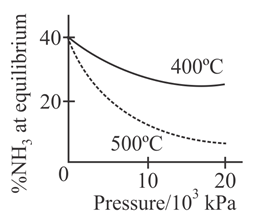
The percentage of ammonia obtainable, if equilibrium were to be established during the Haber process, is plotted against the operating pressure for two temperatures and Which of the following correctly represents the two graphs ?

Important Questions on Equilibrium
For the synthesis of from the Haber process starting with stoichiometric amount of and the attainment of equilibrium is predicted by which curve :-
Equilibrium constant for the reaction
is at
if one mole of placed in a container, what is the weight of formed at equilibrium.
Which of the following statements(s) is/are incorrect:
Statement of contains oxygen atoms
Statement For an aqueous solution to be netral it must have
Statement For the reactions
equilibrium constant is
and equilibrium constant is
then
Statement Whenever -particles emits ratio slightyl increase
Give the correct order of initials (true) of (false) for following statements.
On decreasing pressure to the equilibrium more ice will be formed
At equilibrium, if then
For the reaction is less than
Data for the given reaction (forward)
Then activation energy for the reverse reaction is
