MEDIUM
Earn 100
The pink colour of phenolphthalein in alkaline medium is due to
(a)the acidic form of phenolphthalein.
(b)the anionic form of phenolphthalein.
(c) of the alkali.
(d)The non conjugated structure of phenolphthalein.
33.33% studentsanswered this correctly
Important Questions on Equilibria
HARD
HARD
EASY
HARD
HARD
EASY
MEDIUM
MEDIUM
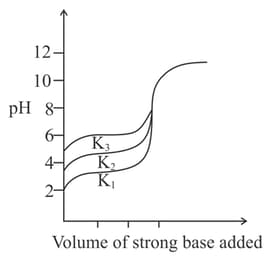
Titration curves for solutions of three weak acids and with ionization constants and respectively are plotted as shown in the figure. Which of the following is/are true?
HARD
MEDIUM
EASY
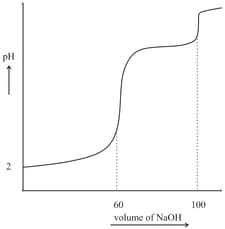
The titration curve for titration of a solution of a diprotic acid with is shown below.
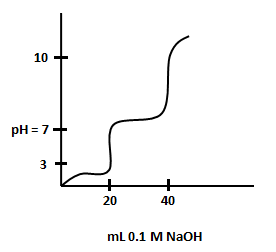
and are approximately
MEDIUM
EASY
HARD
HARD
MEDIUM
Represent the union of two sets by Venn diagram for each of the following.
is a prime number between and
is an odd number between and
HARD
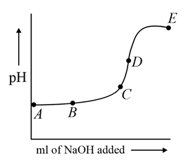
(i) before titration.
(ii) when .
(iii) at point of equivalence.
(iv) When are moles of twice the moles of originally present?
MEDIUM
HARD
HARD

