HARD
JEE Main
IMPORTANT
Earn 100
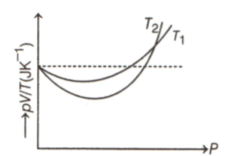
The plot that depicts the behaviour of the mean free time (time between two successive collisions) for the molecules of an ideal gas, as a function of temperature , qualitatively is (graphs are schematic and not drawn to scale)
21.74% studentsanswered this correctly
Important Questions on Thermometry, Thermal Expansion and Kinetic Theory of Gases
HARD
JEE Main
IMPORTANT
Consider two ideal diatomic gases and at some temperature . Molecules of the gas are rigid and have a mass . Molecules of the gas have an additional vibrational mode and have a mass . The ratio of the specific heats ( and ) of gas and respectively is
HARD
JEE Main
IMPORTANT
A gas mixture consists of moles of oxygen and moles of argon at temperature . Considering only translational and rotational modes, the total internal energy of the system is
HARD
JEE Main
IMPORTANT
A vertical closed cylinder is separated into two parts by a frictionless piston of mass and of negligible thickness. The piston is free to move along the length of the cylinder. The length of the cylinder above the piston is and that below the piston is such that Each part of the cylinder contains moles of an ideal gas at equal temperature . If the piston is stationary, its mass , will be given by (where, is universal gas constant and is the acceleration due to gravity)
MEDIUM
JEE Main
IMPORTANT
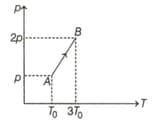
Pressure versus temperature graph of an ideal gas is shown in the given figure. Density of gas at point is , then density of gas at point will be
EASY
JEE Main
IMPORTANT
The figure shows the graph of versus for of hydrogen gas at two different temperatures, where , and represents pressure, volume and temperature respectively. Then, the value of where the curve meet on the vertical axis, is
EASY
JEE Main
IMPORTANT
The wavelength of the radiation emitted by a black body is and Wien's constant is . Then the temperature of the black body will be,
EASY
JEE Main
IMPORTANT
An ideal gas in a closed container is heated so that the final speed of the gas particles, increases by two times the initial speed. If the initial gas temperature is then What will be the final temperature of the ideal gas?
MEDIUM
JEE Main
IMPORTANT
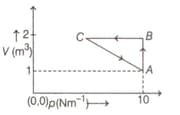
An ideal gas is taken through the cycle as shown in the figure. If the net heat supplied to the gas in the cycle is . The magnitude of work done during the process is