EASY
Earn 100
The possible number of geometrical isomers for octahedral Complex cannot be two.
(a)True
(b)False
50% studentsanswered this correctly
Important Questions on Coordination Chemistry
EASY
HARD
Number of stereoisomers possible for the octahedral complexes and respectively, are:
[-ethylenediamine]
HARD
HARD
(a)
(b) and
(c) is:
HARD
EASY
MEDIUM
The number of stereo isomers possible for is __________.
[ oxalate]
MEDIUM
MEDIUM
Note: and are unidentate neutral and unidentate monoanionic ligands, respectively.
HARD
EASY
EASY
MEDIUM
Three arrangements are shown for the complex, Which one is the wrong statement-
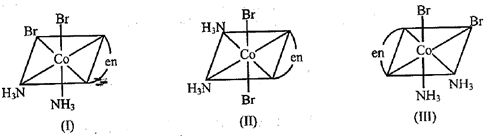
MEDIUM
EASY
MEDIUM
EASY
MEDIUM
MEDIUM
Both geometrical and optical isomerisms are shown by
MEDIUM
will form how many geometrical isomers?

