EASY
JEE Main
IMPORTANT
Earn 100
The potential energy curve for the molecule as a function of internuclear distance is:
(a)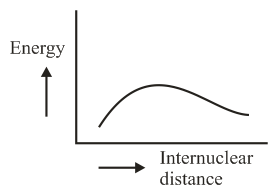

(b)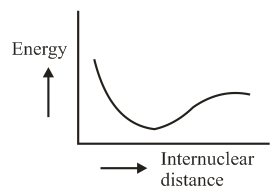

(c)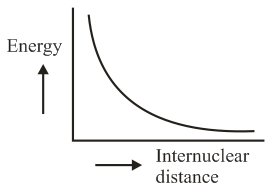

(d)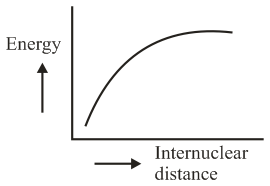

66.67% studentsanswered this correctly

Important Questions on Chemical Bonding and Molecular Structure
MEDIUM
JEE Main
IMPORTANT
The reaction in which the hybridisation of the underlined atom is affected is
EASY
JEE Main
IMPORTANT
The intermolecular potential energy for the molecules , , and given below suggests that:
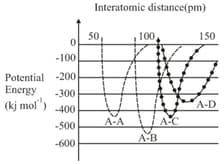
MEDIUM
JEE Main
IMPORTANT
The species in which the N atom is a state of sp hybridization is:
HARD
JEE Main
IMPORTANT
According to molecular orbital theory, the number of unpaired electron(s) in is _________ .
MEDIUM
JEE Main
IMPORTANT
Consider the following molecules and statements related to them:
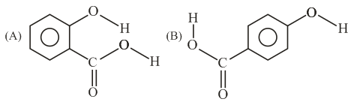
(a) (B) is more likely to be crystaline than (A)
(b) (B) has higher boiling point than (A)
(c) (B) dissolves more readily than (A) in water
Identify the correct option from below:
MEDIUM
JEE Main
IMPORTANT
If the boiling point of is , and the boiling point of will be :
MEDIUM
JEE Main
IMPORTANT
Of the species, and the one with minimum bond strength is :
EASY
JEE Main
IMPORTANT
Which of the following species is not paramagnetic?
