HARD
Earn 100
The potential energy diagram for four reactions are given below
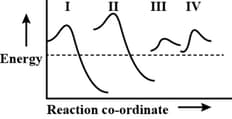
Which one of the following statements about these diagrams is incorrect?

(a) has the largest rate constant for an exothermic reaction
(b) has the smallest rate constant for the reverse reaction
(c) will have the most rapid establishment of equilibrium
(d) has the largest rate constant for an endothermic reaction
50% studentsanswered this correctly
Important Questions on Chemical Kinetics
EASY
EASY
EASY
EASY
HARD
EASY
HARD
MEDIUM
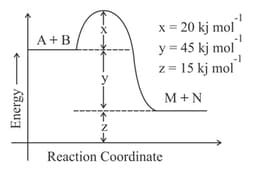
The energy of activation in is ________ . (Nearest integer)
[Given : ]
MEDIUM
HARD
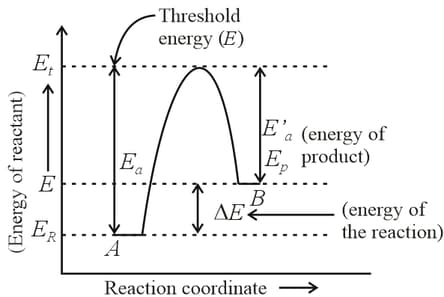
For a reversible reaction, , which one of the following statement is wrong from the given energy profile diagram?
HARD
Define activation energy.
HARD
It was found that the is decreased by in the presence of catalyst. If the rate remains unchanged, the activation energy for catalysed reaction is (Assume pre-exponential factor is same)
MEDIUM
EASY
MEDIUM
in
is equal to __________ . (Integer answer)
MEDIUM
A first order reaction is completed in minutes at and in minutes at . Calculate the activation energy of the reaction.
().
MEDIUM
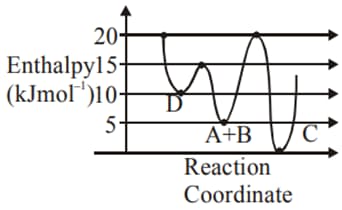
Consider the potential energy diagrams of reactions (I) and (II) given below, predict which reaction will go faster and why ?

HARD
MEDIUM
MEDIUM
Identify the incorrect statement.