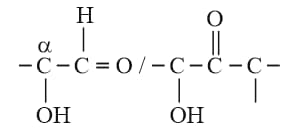The primary structure of proteins contains

Important Points to Remember in Chapter -1 - Biomolecules from Embibe Experts Gamma Question Bank for Engineering Chemistry Solutions
1. Carbohydrates:
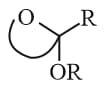
Polyhydroxy aldehyde or Ketones are known as Carbohydrates.
(i) Monosaccharide : single unit, cannot be hydrolysed: Glucose; fructose
(ii) Disaccharide (by glycosidic linkage)
(iii) Polysaccharides: Contain more than two monosaccharide units
: Starch & cellulose
| Types of sugar | |||
| Give Test | Reducing sugar | Non-Reducing sugar | |
|
(i) |
Tollen's Reagent | test | test |
|
(ii) |
Fehling Reagent | test | test |
|
(iii) |
Benedict Test | test | test |
|
(iv) |
Mutarotation | Yes | No |
|
(v) |
Functional Unit |
Hemiacetal Hemiketal |
Acetal
Ketal |
|
(vi) |
Example |
All monosaccharide Glucose, fructose, mannose, galactose, Disaccharide: maltose; lactose |
Disaccharide: Sucrose Polysaccharide: starch, cellulose |
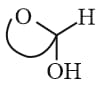
(iv) Mutarotation: When either form is placed in solution it slowly forms the other via open chain aldehyde form & gradual change in specific rotation until specific value is reached.
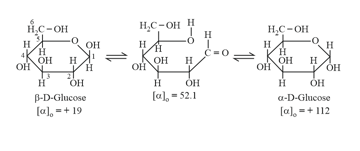
(v) Anomers:
Differ in configuration at carbon due to hemi (acetal or ketal) ring formation. The new symmetric carbon is referred to as Anomeric carbon.
(vi) Epimers:
Diastereomers which differ in conformation at one chiral carbon [maltose & glucose (epimers carbon is )]
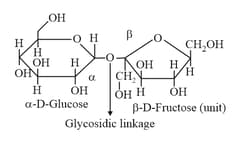
(vii) Sucrose:
(viii) Maltose:
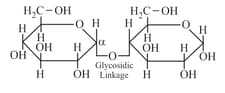
Two - -Glucose units are connected by glycosidic linkage.
(ix) Starch: (Amylose & Amylopectin)
(a) Amylose: (Straight Chain):
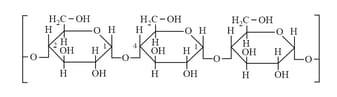
Soluble in & give blue colour with
(b) Amylopectin (Branch chain)
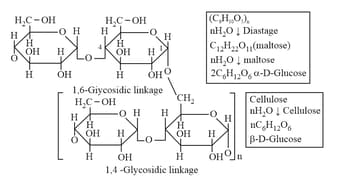
(x) Cellulose: (Straight chain - -Glucose unit):
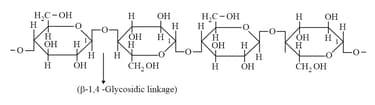
Chemical reactions of Glucose:
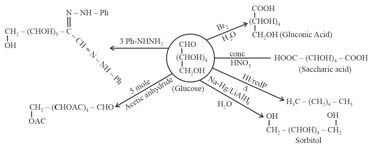
2. Amino acids:
(i) Proteins are made up of amino acids. All naturally occurring amino acids are α-amino acids.
(ii) Amino acids have amphoteric character.
(iii) A free amino group is basic, a free carboxyl group is acidic.
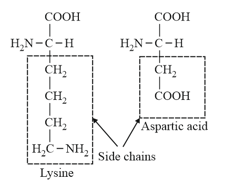
(iv) Lysine and Arginine are basic amino acids, because they carry two amino groups and one carboxyl group.
(v) Glutamic acid (glutamate) and Aspartic acid (aspartate) contain one amino and two carboxyl groups each and are classified as acidic amino acids.
(vi) Alanine, glycine. valine and phenylalanine are neutral amino acids, as these contain one amino and one carboxyl group.
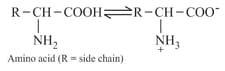
Side chain of a basic and an acidic amino acid.
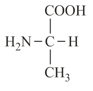 |
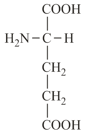 |
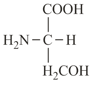 |
| Alanine (non-polar side chain) | Glutamic acid (polar side chain) | Serine (polar side chain) |
3. Proteins:
(i) Proteins are polyamides formed from amino acids.
(ii) The carbon atom of the amino acids is asymmetric and shows optical isomerism (stereo).
(iii) Proteins consist mainly of isomers of amino acids. There are commonly occurring amino acids in proteins. Amino acids form zwitter ion.
(iv) Lack of essential amino acids in diet can cause diseases such as Kwashiorkor.
4. Peptide Bond and Structure of Proteins:
(i) Amino acids are joined together by an amide linkage called peptide bond.
(ii) Proteins are long polymers of amino acids linked by peptide bonds (polypeptides).
(iii) The sequence in which the amino acids are arranged in a protein is called the primary structure.
(iv) The secondary structure arises due to the regular folding of the backbone of the polypeptide chain due to intramolecular hydrogen bonding in α-helix and intermolecular hydrogen bonding in β-plated sheet between the carboxyl and amino groups. Lead to the formation of α -helix and β-pleated sheet.
(v) Tertiary Structure of Proteins: The tertiary structure of proteins represents overall folding of the polypeptide chain i.e., further folding of the secondary structure. It gives rise to two major molecular shapes viz fibrous and globular. The main forces which stabilise the and structures of proteins are hydrogen bonds, disulphide linkages, van der Waals and electrostatic forces of attraction.
(vi) Quaternary structure of proteins: Some of the proteins are composed of two or more polypeptide chains referred to as sub-units. The spatial arrangement of these subunits with respect to each other is known as quaternary structure.