EASY
AP EAPCET
IMPORTANT
Earn 100
The rate equation for a first-order reaction is given by . A straight line with positive slope is obtained by plotting ( Initial concentration of the reactant, concentration of the reactant at time )
(a) Vs time
(b) Vs time
(c) Vs time
(d) Vs time
67.39% studentsanswered this correctly

Important Questions on Chemical Kinetics
MEDIUM
AP EAPCET
IMPORTANT
If the rate constant for a first order reaction is , find the time required to reduce of the reactant to .
EASY
AP EAPCET
IMPORTANT
For an elementary reaction, , the is minutes. In what period of time would the concentration of be reduced to of its original concentration?
MEDIUM
AP EAPCET
IMPORTANT
For a first order reaction , the concentrations vs time plot is as shown. The half-life of the reaction is
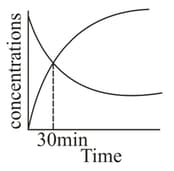
EASY
AP EAPCET
IMPORTANT
For zero order reaction, a plot of versus will be
