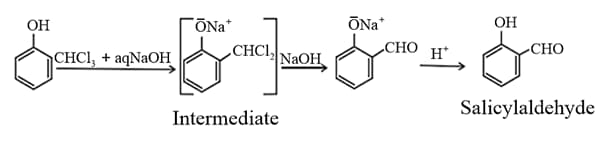
The reaction between phenol and chloroform in the presence of aqueous is

Important Points to Remember in Chapter 11 - Alcohols, Phenols and Ethers from Embibe Experts Achieve CUET (UG) Chemistry Practice Book Solutions
(i) From alkenes :
(a) acid catalysed hydration
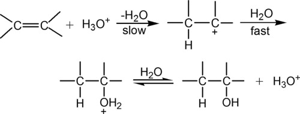
(b) hydroboration–oxidation :
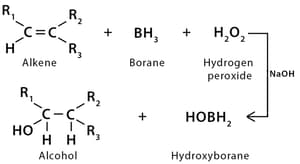
(ii) From carbonyl compounds :
(a) By reduction of aldehydes and ketones
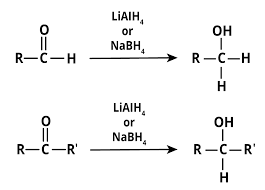
(b) By reduction of carboxylic acids and esters
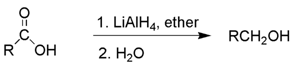
(c) From Grignard reagents :
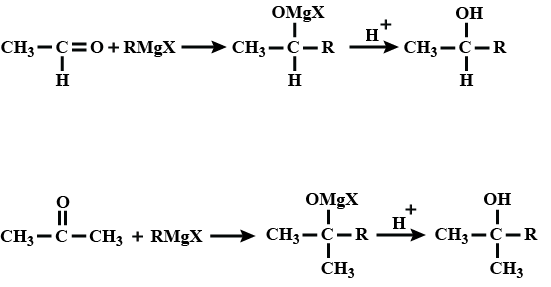
2. Preparation of Phenols
(i) From haloarenes:
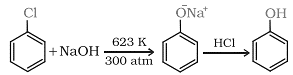
(ii) From benzenesulphonic acid:
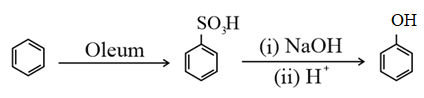
(iii) From diazonium salts:
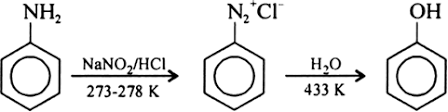
(iv) From cumene:
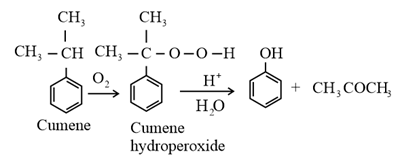
3. Chemical properties of alcohols and phenols:
(i) Alcohols are versatile compounds.
(ii) Electron withdrawing groups in phenol increase its acidic strength and electron releasing groups decrease its acidic strengthacidic strength.
(iii) Alcohols undergo nucleophilic substitution with hydrogen halides to yield alkyl halides.
(iv) Dehydration of alcohols gives alkenes.
(v) Primary alcohols on oxidation yield aldehydes with mild oxidising agents and carboxylic acids with strong oxidising agents while secondary alcohols yield ketones.
(vi) The presence of group in phenols activates the aromatic ring towards electrophilic substitution and directs the incoming group to ortho and para positions due to resonance effect.
(vii) In presence of sodium hydroxide, phenol generates phenoxide ion which is even more reactive than phenol. Thus, in alkaline medium, phenol undergoes Kolbe’s reaction.
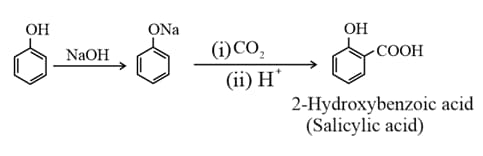
(viii) Reimer-Tiemann reaction of phenol yields salicylaldehyde.
(ix) Phenol is converted to benzene on heating with zinc dust.
(x) Oxidation of phenol with chromic acid produces benzoquinone.
4. Ethers:
Ethers may be prepared by:
(a) dehydration of alcohols :
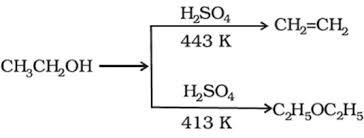
(b) Williamson synthesis:

5. Chemical properties of ethers:
(i) The bond in ethers can be cleaved by hydrogen halides.
(ii) Halogenation of anisole (Electrophilic substitution) :
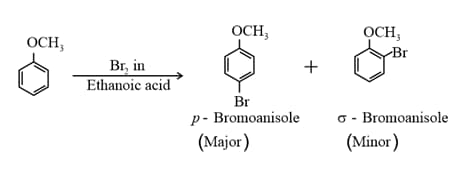
(iii) Friedel-Crafts reaction: Anisole undergoes Friedel-Crafts reaction. The alkyl and acyl groups are introduced at ortho and para positions by reaction with alkyl halide and acyl halide in the presence of anhydrous aluminium chloride (which is a Lewis acid) as catalyst.
(iv) Anisole reacts with a mixture of concentrated sulphuric and nitric acids to yield a mixture of ortho and para nitroanisole.
