EASY
Earn 100
The reaction of cyclopentanone with diazomethane gives
(a)Cyclohexanone
(b)Cyclohexane
(c)Hexane
(d)Hexanone
50% studentsanswered this correctly
Important Questions on Carbonyl Compounds and Carboxylic Acids
MEDIUM
The major product in the following reaction is:
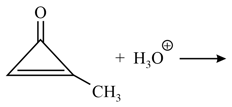
HARD
Columns 1, 2 and 3 contain starting materials, reaction conditions and type of reactions respectively.
| Column 1 | Column 2 | Column 3 |
| (I) Toulene | (i) | (P) Condensation |
| (II) Acetophenone | (ii) | (Q) Carboxylation |
| (III) Benzaldehyde | (iii) | (R) Substitution |
| (IV) Phenol | (iv) | (S) Haloform |
MEDIUM
In the following reaction
carbonyl compound
Rate of the reaction is the highest for:
MEDIUM
For the reaction-
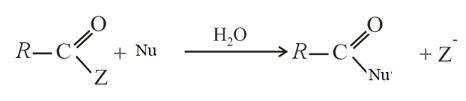 rate of reaction is faster, when is-
rate of reaction is faster, when is-
HARD
The major product of the following reaction is:
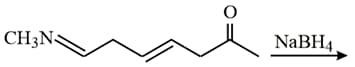
MEDIUM
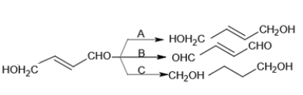
The reagents and respectively are
MEDIUM
The major product of the following reaction is:
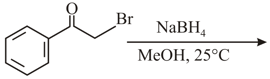
HARD
Benzaldehyde can be converted to benzyl alcohol in concentrated aqueous solution using
HARD
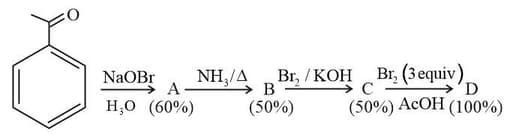
In the following reaction sequence, the amount of (in g) formed from moles of acetophenone is ____.
(Atomic weights in The yield (%) corresponding to the product in each step is given in the parenthesis)
EASY
Reaction of a carbonyl compound with one of the following reagents involves nucleophilic addition followed by elimination of water. The reagent is:
MEDIUM
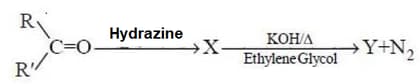
Identify Y in the above sequence of reactions
MEDIUM
Which of the following reagents would distinguish cis-cyclopenta-1, 2-diol from the trans-isomer?
MEDIUM
The compound that does not undergo haloform reaction is
EASY
Which is the most suitable reagent for the following conversion?

MEDIUM
Haloform reaction with and will be responded by
MEDIUM
A compound forms a phenylhydrazone and gives negative Tollen's test and a positive Iodoform reaction. It also gives -pentane on reduction. The compound is
EASY
The most reactive to nucleophilic attack at the carbonyl group is
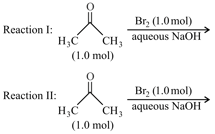
HARD
After completion of the reactions (I and II), the organic compound(s) in the reaction mixtures is(are):
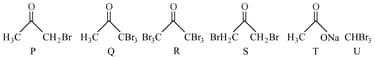
HARD
The test performed on compound and their inferences are:
| Test | Inference |
| - test | Coloured precipitate yellow |
| Iodoform test | Yellow precipitate |
| Azo-dye test | No dye formation |
Compound is:
HARD
Explain the mechanism of aldol addition reaction. Mention two uses of carboxylic acids.

