EASY
Earn 100
The reaction of lead with Copper Chloride solution is an example for which type of chemical reaction? Why? Write the balanced chemical equation for this reaction.
Important Questions on Metals and Non-Metals
EASY
EASY
Observe the diagram showing a copper rod kept immersed in silver nitrate solution.
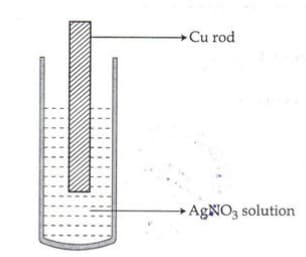
a. What is the colour change of the solution?
b. Write the balanced chemical equation for the reaction.
MEDIUM
Answer the following:
Why are highly reactive metals such as Aluminium is used as reducing agents?
MEDIUM
HARD
EASY
EASY
(a) What is the change that can be noticed on the iron nail after a while?
(b) Write down the chemical equation of the oxidation reaction occurs here?
EASY
Which of the following set of reactions will NOT occur?
I.
II.
III.
IV.
MEDIUM
Food cans are coated with tin and not with zinc because
MEDIUM
MEDIUM
MEDIUM
MEDIUM
MEDIUM
HARD
MEDIUM
EASY
I. Metal H does not react with dilute .
II. Metal K reacts with warm water.
III. Metal L does not react with water but displaces metal H from its aqueous salt solution.
IV. Metal M reacts with cold water.
Choose the correct decreasing order of reactivity of these metals amongst the following.
HARD
EASY
Arrange the following according to the instructions given in bracket:
. (In the increasing order of the reactivity)
MEDIUM

