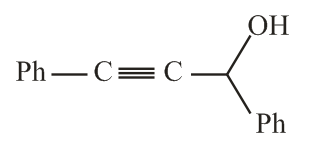
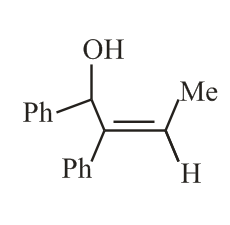
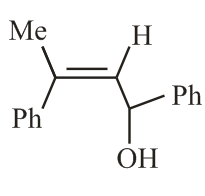
The reaction of phenylacetylene with one equivalent of methyl magnesium bromide followed by reaction with benzaldehyde provides
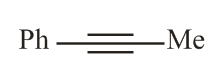

Important Points to Remember in Chapter -1 - Alcohols, Phenols and Ethers from Embibe Experts Gamma Question Bank for Engineering Chemistry Solutions
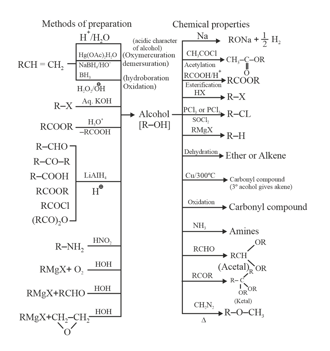
1. Alcohols Preparations and Chemical Properties:
2. Oxidation Reactions of Alcohols
Summary of Oxidation:
| Reagent/Alcohol | 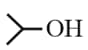 |
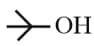 |
||
| (1) | or | 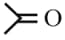 |
||
| (2) | in Solvent |  |
||
| (3) |  |
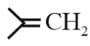 |
||
| (4) |  |
|||
| (5) |  |
|||
| (6) | in water |  |
Note: is a regioselective reagent for the oxidation of only allylic and benzylic alcohols into their respective carbonyl compounds.
3. Physical Properties
(i) Nature of alcohol:
(a) Alcohols are poisonous in nature. Its poisonous character increases with the increment in molecular mass or branching. Ethanol is an exception, which is non-poisonous in nature. Methanol causes blindness.
(b) Isopropyl alcohol is called rubbing alcohol.
(c) Cholesterol is also an alcohol; it causes a heart attack. Hence, it is also called notorious alcohol.
(d) Ethanol is a liquid while glucose is a solid due to more intermolecular -bonding in glucose.
(e) Alcohols are neutral substances towards the litmus paper test.
(f) Lower members containing up to carbon atoms are liquids.
(g) The higher members are solids and are almost odourless.
(h) They have a distinctive smell and a burning taste.
(ii) Boiling point:
(a) Boiling point ∝ Molecular mass
(b) The boiling point of alcohols in water increases as the extent of hydrogen bonding increases.
(c) The boiling point of alcohols are higher than ethers of comparable molecular masses as intermolecular hydrogen bonding is present in alcohols.
(d) Order of Boiling point:
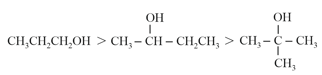
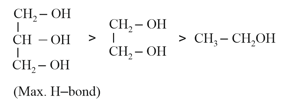
(iii) Solubility in water:
(a) The lower alcohols are soluble in water, and the solubility diminishes as the molecular mass increases.
Solubility Number of branches
(b) Their solubility in water is to be expected since the oxygen atom of the hydroxyl group in alcohols can form hydrogen bonds with water molecules.
(c) The solubility of alcohols in water increases as the extent of hydrogen bonding increases.
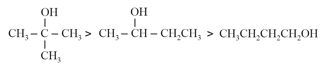
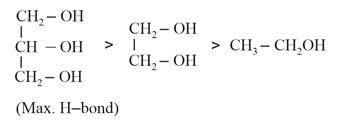
4. Differences between Primary, Secondary, and Tertiary Alcohols:
Oxidation method:
(i) Dichromate test:
(a)
(b)
(c)
(ii) By catalytic oxidation/dehydrogenation:
When the vapours of alcohol are passed over hot metallic at , limited oxidation takes place.
(a) A primary alcohol gives an aldehyde on oxidation.
(b) A secondary alcohol gives a ketone.
(c) A tertiary alcohol gives an alkene (dehydration takes place in tertiary alcohols).
5. Lucas Test:
A mixture of (anhydrous Concentrated ) is called Lucas Reagent.
(i) A tertiary alcohol gives a white precipitate with Lucas reagent in seconds only.
(ii) A secondary alcohol takes minutes.
(iii) A primary alcohol does not give white precipitate at room temperature.
(iv) Allyl alcohol reacts as rapidly as a tertiary alcohol but remains in the solution.
6. Victor Meyer Test:
This test is also known as (Red, Blue, Colourless) test.
(i) 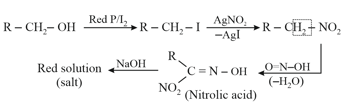
(ii) 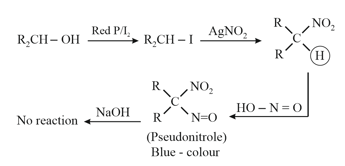
(iii) 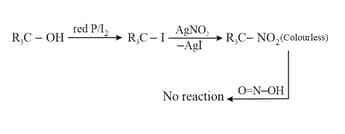
7. Chemical Properties and Method of Preparation of Glycol:
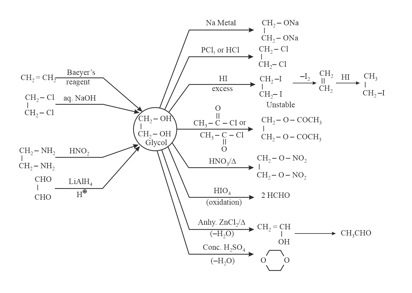
8. Chemical Properties and Method of Preparation of Glycerol:
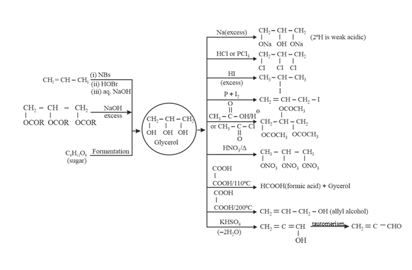
9. Preparation and Chemical Properties of Ethers:
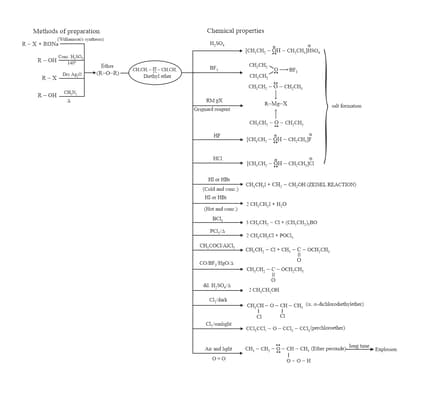
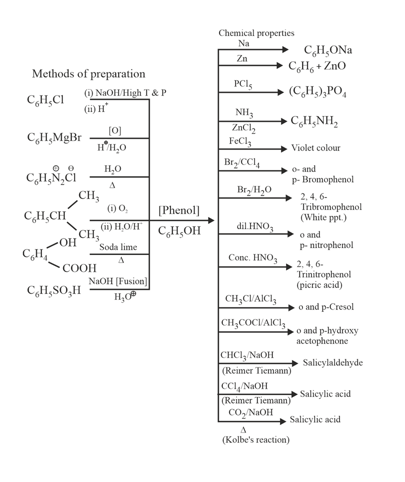
10. Preparation and Chemical Properties of Phenols: