MEDIUM
AS and A Level
IMPORTANT
Earn 100
The sketch graph shows the successive ionization energies of aluminium.
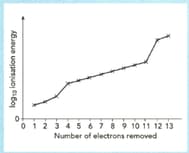
Explain how the graph provides evidence for the existence of three electrons shells in an aluminium atom.

Important Questions on Electrons in Atoms
MEDIUM
AS and A Level
IMPORTANT
Write an equation, including state symbols, to represent the ionization energy aluminium.
HARD
AS and A Level
IMPORTANT
Write the electronic configuration of an aluminium ion, using notation.
MEDIUM
AS and A Level
IMPORTANT
The table shows the first ionization energies,,in , of the elements in the period of the periodic table.
| Element | Na | Mg | Al | Si | P | S | Cl | Ar |
| 494 | 736 | 577 | 786 | 1060 | 1000 | 1260 | 1520 |
Explain why there is a general increase in the value of across the period.
MEDIUM
AS and A Level
IMPORTANT
The table shows the first ionization energies,,in , of the elements in the period of the periodic table.
| Element | Na | Mg | Al | Si | P | S | Cl | Ar |
| 494 | 736 | 577 | 786 | 1060 | 1000 | 1260 | 1520 |
Write the electronic configuration for argon using notation.
MEDIUM
AS and A Level
IMPORTANT
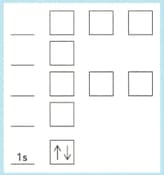
Copy and complete the diagram below for the electrons in phosphorus by:
Adding labels for the other sub-shell.
MEDIUM
AS and A Level
IMPORTANT
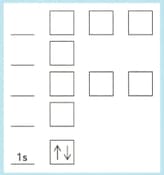
Copy and complete the diagram below for the electrons in phosphorus by:
Showing how the electrons are arranged.
MEDIUM
AS and A Level
IMPORTANT
Predict a value for the first ionization energy for potassium, which has one more proton than argon.
EASY
AS and A Level
IMPORTANT
Draw diagrams to show the shape of p-orbital.
