EASY
AS and A Level
IMPORTANT
Earn 100
The specific latent heat of fusion of water is . Calculate the energy needed to change of ice into water at . Suggest why the answer is much smaller than .

Important Questions on Thermal Physics
EASY
AS and A Level
IMPORTANT
A sample of alcohol is heated with a heater until it boils. As it boils, the mass of the liquid decreases at a rate of per minute. Assuming that 80% of the energy supplied by the heater is transferred to the alcohol, estimate the specific latent heat of vaporisation of the alcohol. Give your answer in .
EASY
AS and A Level
IMPORTANT
List all the ideas in Thermal Physics that are associated with an increase in temperature.
EASY
AS and A Level
IMPORTANT
What strategies could you use to make sure you understand these?
EASY
AS and A Level
IMPORTANT
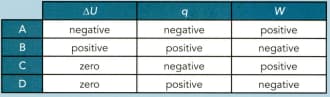
The first law of thermodynamics can be represented by the expression:
An ideal gas is compressed at constant temperature.
Which row shows whether and are negative, positive or zero during the change?
EASY
AS and A Level
IMPORTANT
What is the internal energy of an object?
EASY
AS and A Level
IMPORTANT
Describe the changes to the kinetic energy, the potential energy and the total internal energy of its molecules as ice melts into water.
Ice melts at .
EASY
AS and A Level
IMPORTANT
Describe the changes to the kinetic energy, the potential energy and the total internal energy of the molecules of a block of ice as:
The temperature of the water rises from to room temperature.
EASY
AS and A Level
IMPORTANT
Explain, in terms of kinetic energy, why the temperature of a stone increases when it falls from a cliff and lands on the beach below.
