HARD
Earn 100
The state of hybridisation of S in is
(a) and has a lone pair of electron
(b) and has tetrahedral structure
(c) and has a trigonal bipyramidal structure
(d) and has an octahedral structure
100% studentsanswered this correctly
Important Questions on Chemical Bonding and Molecular Structure
EASY
EASY
MEDIUM
MEDIUM
MEDIUM
MEDIUM
EASY
EASY
EASY
EASY
EASY
MEDIUM
EASY
EASY
EASY
The hybridisations of and shown in the following compound
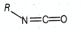
respectively, are
EASY
In the following molecules,
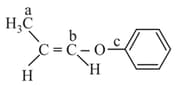
Hybridisation of carbon and respectively are :
EASY
EASY
EASY
EASY

