MEDIUM
JEE Main
IMPORTANT
Earn 100
The strength of an aqueous solution is most accurately determined by titrating: (Note: consider that an appropriate indicator is used)
(a) in a pipette and aqueous oxalic acid in a burette
(b) in a burette and aqueous oxalic acid in a conical flask
(c) in a burette and concentrate in a conical flask
(d) in a volumetric flask and concentrated in a conical flask
76.92% studentsanswered this correctly

Important Questions on Equilibrium
MEDIUM
JEE Main
IMPORTANT
The stoichiometry and solubility product of a salt with the solubility curve given below is, respectively:
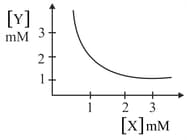
MEDIUM
JEE Main
IMPORTANT
For the following reaction, equilibrium constant are given:
The equilibrium constant for the reaction, is:
MEDIUM
JEE Main
IMPORTANT
If solubility product of is denoted by and its molar solubility is denoted by , then which of the following relation between and is correct?
HARD
JEE Main
IMPORTANT
Which of the following lines correctly show the temperature dependence of equilibrium constant for an exothermic reaction?
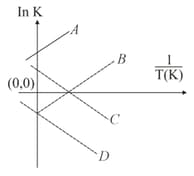
HARD
JEE Main
IMPORTANT
An aqueous solution contains an unknown concentration of . When solution of is added, just begins to precipitate. The final volume is . The solubility product of is . What is the original concentration of ?
MEDIUM
JEE Main
IMPORTANT
Which of the following are Lewis acids?
HARD
JEE Main
IMPORTANT
An aqueous solution contains and . If the equilibrium constant for the formation of from is and that of from ions is , then, the concentration of ions in the aqueous solution is:
MEDIUM
JEE Main
IMPORTANT
Which of the following salts is the most basic in aqueous solution?
