HARD
Earn 100
The structure of can be best described as
(a)
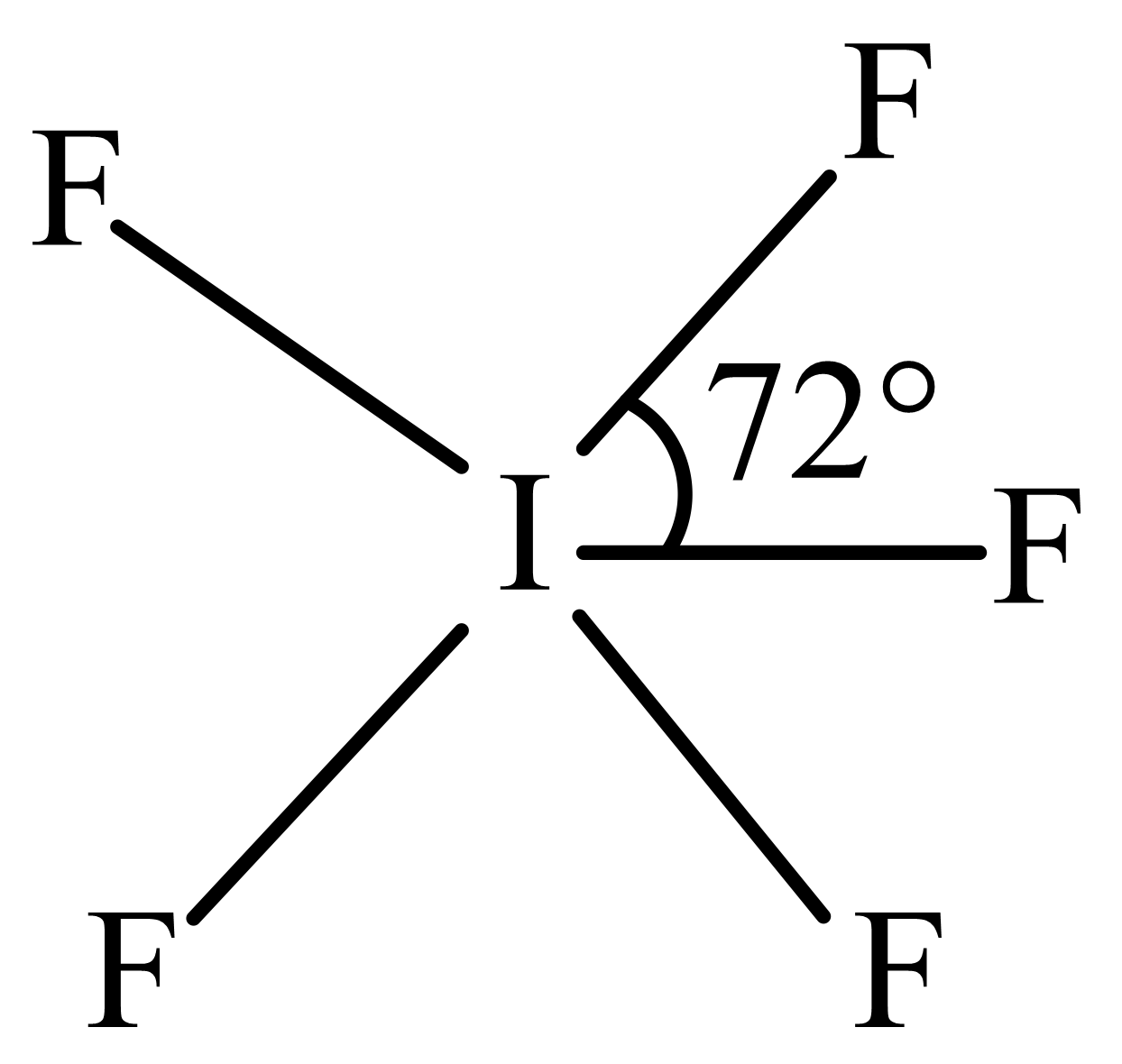
(b)
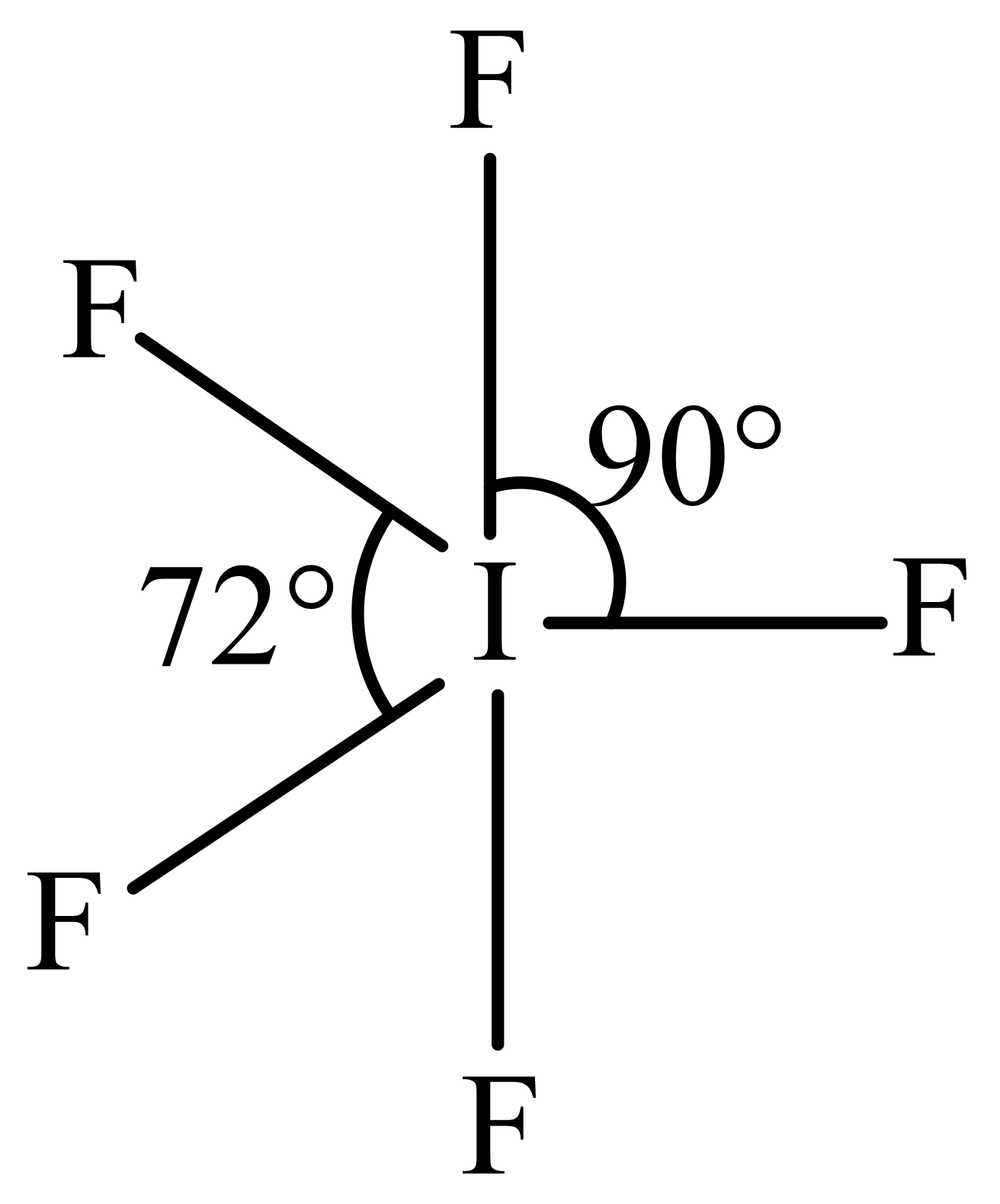
(c)

(d)None of these
50% studentsanswered this correctly
Important Questions on Chemical Bonding
HARD
EASY
MEDIUM
HARD
MEDIUM
MEDIUM
EASY
EASY
MEDIUM
MEDIUM
MEDIUM
EASY
MEDIUM
MEDIUM
HARD
EASY
EASY
Which of the following statements is false:
MEDIUM
EASY
EASY

