EASY
Earn 100
The structure of propene is:
(a)
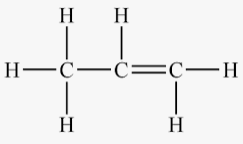
(b)
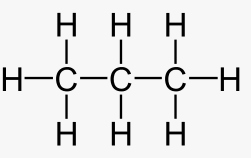
(c)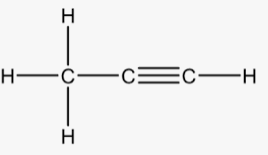

(d)
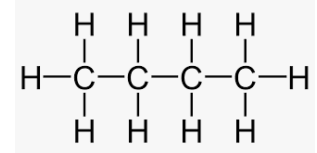
50% studentsanswered this correctly
Important Questions on Carbon Compounds
MEDIUM
EASY
HARD
Complete the following table:
| Straight chain of Carbon compounds | Structural formula | Molecular formula | Name |
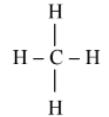 |
Methane | ||
| _____ | _____ | Ethane | |
| _____ | _____ | ||
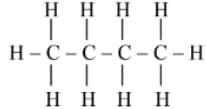 |
_____ | _____ |
EASY
EASY
EASY
Draw the structure of the compound of carbon given below.
Chloropropane.
MEDIUM
Draw the structures of the following.
(i) Hexanal
(ii) Propanone
HARD
HARD
MEDIUM
MEDIUM
MEDIUM
Match the pairs.
| Group A | Group B |
| a. | 1. Unsaturated hydrocarbon |
| b. | 2. Molecular formula of an alcohol |
| c. | 3. Saturated hydrocarbon |
| d. | 4. Triple bond |
MEDIUM
MEDIUM
MEDIUM
MEDIUM
EASY
MEDIUM
HARD
HARD

