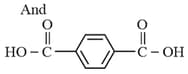
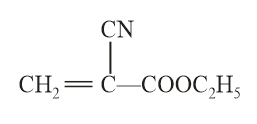
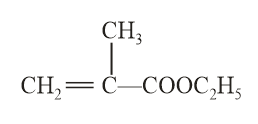
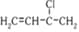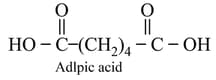The structure of the monomer used for the preparation of plexiglass is

Important Points to Remember in Chapter -1 - Polymers from Embibe Experts Gamma Question Bank for Engineering Chemistry Solutions
1. Classification Based on the Source:
(i) Natural polymers:
These polymers are found in plants and animals. Examples are proteins, cellulose, starch, some resins and rubber.
(ii) Semi-synthetic polymers:
Cellulose derivatives as cellulose acetate (rayon) and cellulose nitrate, etc., are the usual examples of this subcategory.
(iii) Synthetic polymers:
A variety of synthetic polymers as plastic (polythene), synthetic fibres (nylon ) and synthetic rubbers (Buna – ) are the examples of manmade polymers extensively used in daily life as well as in industry.
2. Classification Based on the Structure of Polymers:
(i) Linear polymers:
These polymers consist of long and straight chains. The examples are high-density polythene, polyvinyl chloride, etc. These are represented as:
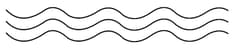
(ii) Branched chain polymers:
These polymers contain linear chains having some branches. Example is low density polythene.
These are depicted as follows:
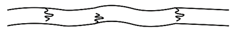
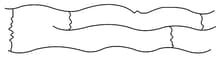
(iii) Cross linked or Network polymers:
These are usually formed from bi-functional and trifunctional monomers and contain strong
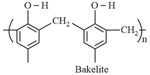
covalent bonds between various linear polymer chains. Examples are Bakelite, melamine, etc. These polymers are depicted as follows:
3. Classification Based on Mode of Polymerization:
(i) Addition polymers:
(a) The addition polymers are formed by the repeated addition of monomer molecules possessing double or triple bonds, for example, the formation of polythene from ethene and polypropene from propene.
(b) However, the addition polymers formed by the polymerisation of a single monomeric species are known as homopolymers, for example, polythene.
(c) The polymers made by addition polymerisation from two different monomers are termed as copolymers, for example, Buna- , Buna- , etc.
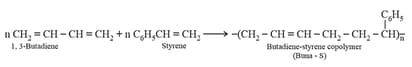
(ii) Condensation polymers:
(a) The condensation polymers are formed by repeated condensation reactions between two different bi-functional or tri-functional monomeric units.
(b) In these polymerisation reactions, the elimination of small molecules such as water, alcohol, hydrogen chloride, etc., takes place.
(c) The examples are terylene (Dacron), nylon nylon etc. For example, nylon is formed by the condensation of hexamethylenediamine with adipic acid.
4. Classification Based on Molecular Forces:
(i) Elastomers:
(a) These are rubber - like solids with elastic properties.
(b) In these elastomeric polymers, the polymer chains are held together by the weakest intermolecular forces.
(c) These weak binding forces permit the polymer to be stretched.
(d) A few 'crosslinks' are introduced in between the chains, which help the polymer to retract to its original position after the force is released as in vulcanised rubber. The examples are buna- . buna- , neoprene, etc.
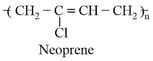
(ii) Fibres:
(a) Fibres are the thread forming solids which possess high tensile strength and high modulus.
(b) These characteristics can be attributed to the strong intermolecular forces like hydrogen bonding.
(c) These strong forces also lead to close packing of chains and thus impart crystalline nature.
(d) The examples are polyamides (nylon ), polyesters (terylene), etc.
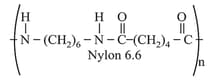
(iii) Thermoplastic polymers:
(a) These are the linear or slightly branched long chain molecules capable of repeatedly softening on heating and hardening on cooling.
(b) These polymers possess intermolecular forces of attraction, intermediate between elastomers and fibres. Some common thermoplastics are polythene, polystyrene, polyvinyl, etc.
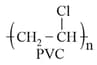
(iv) Thermosetting polymers:
These polymers are cross linked or heavily branched molecules, which on heating undergo extensive cross linking in moulds and again become infusible. These cannot be reused. Some common examples are Bakelite, urea-formaldehyde resins, etc.
5. Type of polymerisation:
(i) Chain Growth Polymerisation or Addition Polymerisation:
(a) It involves formation of reactive intermediates such as free radical, a carbocation or a carbanion.
(b) For this polymerisation, monomers used are unsaturated compounds like alkenes, alkadienes and their derivatives.
(c) Depending upon the nature of the reactive species involved. chain growth polymerisation occurs by the following mechanisms:
Free radical addition polymerisation:
The monomers used are generally monosubstituted alkenes. The most used catalysts are benzoyl peroxide, hydrogen peroxide or t-butyl peroxide etc.
Step I: Chain initiation step: In this step, peroxide undergoes homolytic fission, e.g., benzoyl peroxide on heating produces phenyl initiator free radical.
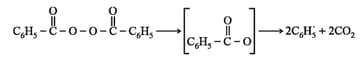
Step II: Chain propagation step: The new free radical adds to another molecules of monomer to form a larger free radical.
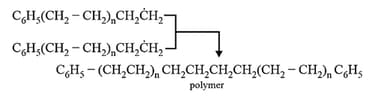
Step III: Chain termination step: There are three ways of chain termination: Coupling reaction, disproportionation reaction, chain transfer reaction. One mode of termination of chain is shown as under:
(ii) Cationic polymerisation:
It involves formation of carbocation which are generated by Lewis acids (like etc.) and protonic acids such as etc. Higher the stability of carbocation intermediate, more is the reactivity of monomers towards cationic addition polymerisation. It involves the following steps:
Step I. Initiation Step
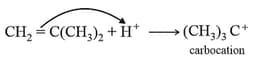
Step II. Propagation
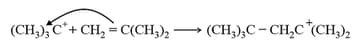
(iii) Anionic polymerisation:
It involves the formation of a carbanion. Steps involved in this process are:
Step I: Initiation Strong bases act as initiator.
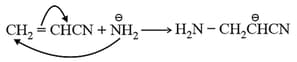
Step II: Propagation
Step III: Termination
(iv) Step Growth Polymerisation:
Condensation polymerisation which occurs in a stepwise manner with the elimination of some smaller molecules like etc., is concerned with the step growth polymerisation, for example, adipic acid and hexamethylenediamine phenol and formaldehyde etc., undergo step Growth Polymerisation.
(v) Distinction Between Chain Growth Polymerisation and Step Growth Polymerisation:
| S. No. | Chain growth polymerisation | Step growth polymerisation |
| 1. | it proceeds by a chain mechanism, characterized by initiation, chain propagation and chain termination. | It proceeds by an equilibrium step mechanism. The step growth process is usually much slower than the chain growth polymerisation. |
| 2. | Only one repeating unit is added at a time. | Any two species present can react with Elimination of some by product. |
| 3. | Reaction mixtures contain only monomers, polymers, and the growing chain. | All the molecular species are present at every stage of polymerisation. |
6. Molecular mass of polymer:
(i) Number-Average Molecular Mass
If molecules have molecular mass each, molecules have molecular mass each,
molecules have molecular mass each and so on,
Number-Average Molecular Mass
If molecules have molecular mass each molecules have molecular mass each,
molecules have molecular mass each and so on,
Then,
(ii) Mass-Average Molecular Mass
Supposing, as before that etc., molecules have molecular mass etc., respectively,
then
It is determined by light scattering and ultracentrifugation method.
(iii) Polydispersity index:
It is the ratio of the mass average molecular mass to the number average molecular mass.
For natural polymers, is usually equal to one which means that they are monodisperse. In other words, such polymers are more homogeneous. On the contrary, synthetic polymers generally have
which means that they are less homogeneous.
7. Some Commercially important Polymers
(i) Addition polymers
| S. No. | Name of Polymer | Abbreviations | Starting Materials | Nature of Polymer | Properties | Applications |
|
(a) Polyolefins |
||||||
|
I. |
Polyethylene Polyethene | Low density photopolymer (branched chain growth |
Transparent, moderate tensile strength, high toughness |
Packing material bags, insulation for electrical wires and cables. Buckets, tubes, houseware pipes, bottles and toys |
||
|
II. |
Polypropylene | Homopolymer, linear, chain growth |
Harder and stronger than polyethene |
Packing of textiles and foods, liners for bags, heat shrinkage wraps, carpet fibres, ropes, automobile mouldings, stronger pipes and bottles. | ||
|
III. |
Polystyrene or Styron |
Photopolymer, linear, chain growth |
Transparent | Plastic toys, household wares, radio and television bodies, refrigerator linings. | ||
|
(b) Polydienes |
||||||
|
I. |
Neoprene |
Chloroprene or –Chloro -butadiene |
Homopolymer, chain growth |
Rubber like, a superior resistant to aerial oxidation, and oils, gasoline etc. |
Horses shoe heels, stoppers. |
|
|
II. |
Buna (Styrene- Butadiene, Rubber) | or |
–butadiene and Styrene |
Copolymer, chain growth | Rubber like, a superior resistant to aerial oxidation, and oils, gasoline etc. | Manufacturer of tyres, rubber soles, waterproof shoes. |
|
(c) Polyacrylates |
||||||
|
I. |
Polymethylmethacrylate (Plexiglas Lucite, Acrylate or Perspex) |
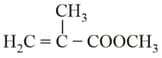 |
Homopolymer |
Hard, transparent. excellent light transmission. Optical clarity better than glass, takes up colours. |
Lenses, light covers, light shades, signboards, transparent domes, skylights, aircraft windows, dentures and plastic jewellery. | |
|
II. |
Polymethacrylate | Homopolymer | Tough, rubber like product. | |||
|
III. |
Polyacrylonitrile Orlon | Homopolymer | Hard, horny, and high melting materials. | Orion is used for making clothes, carpets, blankets, and preparation of other polymers. | ||
|
(d) Polyhalofins |
||||||
|
I. |
Polyvinyl chloride | Homopolymer chain growth | Pliable (easily moulded) |
(i) Plasticised with polyester polymers used in raincoats, handbags, shower curtains, fabrics, shoe soles, vinyl flooring (ii) Good electrical insulator (iii) Hose pipes. |
||
|
II. |
Polytetrafluoroethylene, or Teflon | Homopolymer |
Flexible and inert to solvents, boiling acids even aqua regia, stable up to |
(i) For non-stick utensils coating (ii) Making gaskets, pump packings, valves, seals, non-lubricated bearings. |
||
|
III. |
Polymonochlorotrifluor -ethylene |
Less resistant to heat and chemicals due to presence of chlorine atoms. |
Like those of Teflon. |
(ii) Condensation polymer:
| S.NO. | Name of Polymer | Abbreviations | Starting Materials | Nature of Polymer | Properties | Applications |
|
(a) Polyesters |
||||||
|
I. |
Terylene or Dacron or Mylar |
Ethylene glycol or Ethane –diol
Terephthalic acid or Benzene- dicarboxylic acid |
Copolymer, step growth, linear |
Fibre crease resistant, low moisture |
For wash and wear fabrics, tyre cords, sea belts and sails. | |
|
II. |
Glyptal or Alkyl resin |
Ethylene glycol And
Phthalic acid or Benzene -dicarboxylic acid |
Copolymer, linear step growth |
Thermoplastic, dissolves in suitable solvents |
Paints and lacquers. | |
|
(b) Polyamides |
||||||
|
(i) |
Nylon- |
and Hexamethylenediamine |
Copolymer, linear, step growth | |||
|
(ii) |
Nylon- |
Hexamethylene diamine and Sebacic acid |
Copolymer, linear, step growth |
High tensile strength, abrasions resistant, somewhat elastic |
(i) Textile fabrics, carpets, bristles for brushes. (ii) Substitute of metals in bearings Gears elastic hosiery. Mountaineering ropes, tyre cords, fabrics. |
|
|
(iii) |
Nylon- or Perlon | 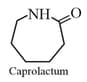 |
Homopolymer linear | Mountaineering ropes, tyre cords, fabrics. | ||
|
(c) Formaldehyde resins |
||||||
|
I. |
Phenol-formaldehyde resin or Bakelite | Phenol and formaldehyde | Copolymer, step growth |
(i) With low degree polymerization for binding glue, wood, varnishes, lacquers. (ii) With high degree polymerisation for combs, for mica |
||
|
II. |
Melamine formaldehyde resin |
Melamine and formaldehyde | Copolymer, step growth | Tough, rubber like product. |
Non-breakable and non-plastic crockery. |
(iii) Biodegradable polymers:
| Name | Monomer | Uses |
| Poly- -hydroxy butyrate-Cohydroxy valerate | 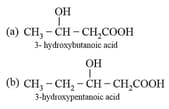 |
As packaging orthopaedic devices and in controlled drug release. |