EASY
AS and A Level
IMPORTANT
Earn 100
The structures of carbon dioxide and silicon dioxide are shown in the diagram below.
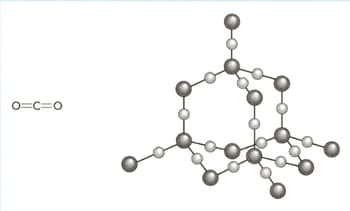
Use your knowledge of structure and bonding to explain the following:
neither carbon dioxide nor silicon(IV) oxide conducts electricity.


Important Questions on States of Matter
EASY
AS and A Level
IMPORTANT
This question is about gases.
What do you understand by the term ideal gas?
EASY
AS and A Level
IMPORTANT
This question is about gases.
Under what conditions do a gas not behave ideally? Explain your answer for one of these conditions.
EASY
AS and A Level
IMPORTANT
This question is about gases.
Helium is a noble gas. It exists as single atoms. Explain why:
helium has a very low boiling point
EASY
AS and A Level
IMPORTANT
This question is about gases.
Helium is a noble gas. It exists as single atoms. Explain why:
helium does not conduct electricity.
EASY
AS and A Level
IMPORTANT
This question is about gases.
A weather balloon contains 0.500 Kg of helium. Calculate the volume of the gas in the balloon at a pressure of
0.500 × 105 Pa and a temperature of -20.0 °C.
(R = 8.31 JK–1mol–1; Ar He = 4.0)
EASY
AS and A Level
IMPORTANT
Water and bromine are both simple molecular substances. Both water and bromine form a lattice structure in the solid-state.
What do you understand by the term lattice?
EASY
AS and A Level
IMPORTANT
Water and bromine are both simple molecular substances.
The boiling point of water is 100°C. The boiling point of bromine is 59°C. Explain the reason for this difference in terms of intermolecular forces.
EASY
AS and A Level
IMPORTANT
Water and bromine are both simple molecular substances.
Use ideas about the kinetic theory to explain what happens when liquid bromine evaporates to form bromine vapour.
