The table shows some properties of four elements. Use the data to answer the following questions. (Assume that steel has similar properties to iron.)
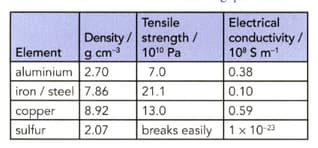
Explain the differences in tensile strength and electrical conductivity of iron and sulfur.


Important Questions on States of Matter
Explain the following property of silicon(IV) oxide by referring to its structure and bonding:
It has a high melting point.
It does not conduct electricity.
It is a crystalline solid.
silicon(IV) oxide is hard.
buckminsterfullerene, C60, is converted from a solid to a gas at a relatively low temperature
Suggest, using ideas of structure and bonding, why:
buckminsterfullerene, C60, is relatively soft.
Four types of structure are:
giant molecular
giant ionic
giant metallic
simple molecular
Give two examples of a giant ionic structure and two examples of a simple molecular structure.
