EASY
AS and A Level
IMPORTANT
Earn 100
The table shows the atomic number and boiling points of some noble gases.
Gas
helium
neon
argon
krypton
xenon
Atomic number
Boiling point /
The structure of xenon trioxide is shown below.
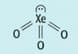
Draw the structure of xenon trioxide to show the partial charges on the atoms and the direction of the dipole in the molecule.


Important Questions on Chemical Bonding
EASY
AS and A Level
IMPORTANT
Electronegativity values can be used to predict the polarity of bonds. Define the term electronegativity.
HARD
AS and A Level
IMPORTANT
Methane, , is a gas at room temperature. Explain why methane is a gas at room temperature.
MEDIUM
AS and A Level
IMPORTANT
Draw a diagram to show the shape of a molecule of methane. On your diagram show a value for the bond angle.
MEDIUM
AS and A Level
IMPORTANT
Perfumes often contain molecules that have simple molecular structures. Explain why.
HARD
AS and A Level
IMPORTANT
When a negatively charged rod is held next to a stream of propanone, , the stream of propanone is attracted to the rod. Draw the full structure of a molecule of propanone and use your diagram to explain why the stream of propanone is attracted to the rod.
MEDIUM
AS and A Level
IMPORTANT
Sodium iodide and magnesium oxide are ionic compounds. Iodine and oxygen are covalent molecules. Draw a dot and cross diagram for magnesium oxide.
MEDIUM
AS and A Level
IMPORTANT
Sodium iodide and magnesium oxide are ionic compounds. Iodine and oxygen are covalent molecules. Draw a dot and cross diagram for oxygen.
HARD
AS and A Level
IMPORTANT
Describe how sodium iodide and iodine differ in their solubility in water. Explain your answer.
