The table shows the atomic number and boiling points of some noble gases.
Gas
helium
neon
argon
krypton
xenon
Atomic number
Boiling point /
Use ideas about forces between atoms to explain this trend in boiling points.

Important Questions on Chemical Bonding
The table shows the atomic number and boiling points of some noble gases.
| Gas | helium | neon | argon | krypton | xenon |
| Atomic number | |||||
| Boiling point / |
Xenon forms a number of covalently bonded compounds with fluorine. Define the terms covalent bond.
The table shows the atomic number and boiling points of some noble gases.
| Gas | helium | neon | argon | krypton | xenon |
| Atomic number | |||||
| Boiling point / |
Xenon forms a number of covalently bonded compounds with fluorine. Draw a dot-and-cross diagram for xenon tetrafluoride, .
The table shows the atomic number and boiling points of some noble gases.
| Gas | helium | neon | argon | krypton | xenon |
| Atomic number | |||||
| Boiling point / |
Xenon forms a number of covalently bonded compounds with fluorine. Suggest a shape for . Explain your answer.
The table shows the atomic number and boiling points of some noble gases.
| Gas | helium | neon | argon | krypton | xenon |
| Atomic number | |||||
| Boiling point / |
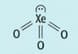
The structure of xenon trioxide is shown below.
By referring to electron pairs, explain why xenon trioxide has this shape.
The table shows the atomic number and boiling points of some noble gases.
| Gas | helium | neon | argon | krypton | xenon |
| Atomic number | |||||
| Boiling point / |
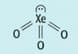
The structure of xenon trioxide is shown below.
Draw the structure of xenon trioxide to show the partial charges on the atoms and the direction of the dipole in the molecule.