MEDIUM
AS and A Level
IMPORTANT
Earn 100
The table shows the first ionization energies,,in , of the elements in the period of the periodic table.
Element
Na
Mg
Al
Si
P
S
Cl
Ar
494
736
577
786
1060
1000
1260
1520
Explain why there is a general increase in the value of across the period.

Important Questions on Electrons in Atoms
MEDIUM
AS and A Level
IMPORTANT
The table shows the first ionization energies,,in , of the elements in the period of the periodic table.
| Element | Na | Mg | Al | Si | P | S | Cl | Ar |
| 494 | 736 | 577 | 786 | 1060 | 1000 | 1260 | 1520 |
Explain why aluminium has a lower value of than magnesium.
MEDIUM
AS and A Level
IMPORTANT
The table shows the first ionization energies,,in , of the elements in the period of the periodic table.
| Element | Na | Mg | Al | Si | P | S | Cl | Ar |
| 494 | 736 | 577 | 786 | 1060 | 1000 | 1260 | 1520 |
Write the electronic configuration for argon using notation.
MEDIUM
AS and A Level
IMPORTANT
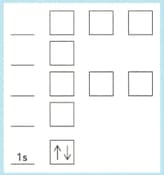
Copy and complete the diagram below for the electrons in phosphorus by:
Adding labels for the other sub-shell.
MEDIUM
AS and A Level
IMPORTANT
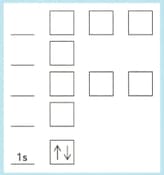
Copy and complete the diagram below for the electrons in phosphorus by:
Showing how the electrons are arranged.
MEDIUM
AS and A Level
IMPORTANT
Predict a value for the first ionization energy for potassium, which has one more proton than argon.
EASY
AS and A Level
IMPORTANT
State the meaning of the term atomic orbital.
EASY
AS and A Level
IMPORTANT
Draw diagrams to show the shape of S-Orbital.
EASY
AS and A Level
IMPORTANT
Draw diagrams to show the shape of p-orbital.
