MEDIUM
Earn 100
The temperature at which ice converts to water is
(a)
(b)
(c)
(d)
100% studentsanswered this correctly
Important Questions on Matter in our Surroundings
MEDIUM
MEDIUM
MEDIUM
When does ice melt at the same temperature (273 Kelvin), which one of the following has more energy?
MEDIUM
HARD
MEDIUM
MEDIUM
HARD
MEDIUM
HARD
MEDIUM
HARD
MEDIUM
MEDIUM
Common salt, water and sand
MEDIUM
MEDIUM
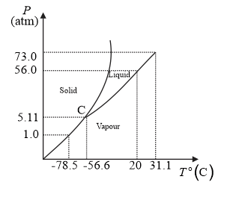
The following questions based on the phase diagram of carbon dioxide:
At what temperature and pressure can the solid, liquid and vapour phases of co-exist in equilibrium?
MEDIUM
Kerosene oil, water and salt
MEDIUM
MEDIUM
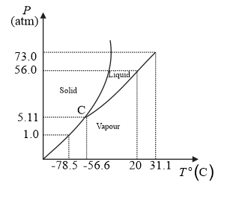
Answer the following questions based on the P–T phase diagram of :
at 1 atm pressure and temperature is compressed isothermally. Does it go through a liquid phase?