The value of and for a gas are R and R. The vapour density of gas is . Its atomic mass will be
Important Questions on Chemical thermodynamics and energetics
(Latent heat of ice is and )
The combination of plots which does not represent isothermal expansion of an ideal gas is




The quantity of heat (in ) required to raise the temperature of of ethanol from to the boiling point and then change the liquid to vapor at that temperature is closest to [Given, boiling point of ethanol . Specific heat capacity of liquid ethanol . Latent heat of vaporisation of ethanol ]
The molar heat capacity for an ideal gas at constant pressure is . The change in internal energy is upon heating it from to . The number of moles of the gas at constant volume is____
(Given: )
The specific heat of a certain substance is . Assuming ideal solution behavior, the energy required (in ) to heat of molal of its aqueous solution from to is closest to :
[Given: molar mass of the substance ; specific heat of water ]

The correct option(s) is (are)

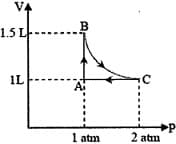
The above diagram represents the thermodynamic cycle of an engine, operating with an ideal mono-atomic gas. The amount of heat, extracted from the source in a single cycle, is:

(R = 8.314 J/mol K) (ln7.5 = 2.01)
of is mixed with of The molar heat of neutralization of this reaction is The increase in temperature in of the system on mixing is The value of is (Nearest integer)
[Given: Specific heat of water
Density of water]
(Assume no volume change on mixing)
[Heat of fusion of ice ; Specific heat of water ]

