EASY
12th West Bengal Board
IMPORTANT
Earn 100
The value of Henry's constant is
(a)greater for gases with higher solubility,
(b)greater for gases with lower solubility.
(c)constant for all gases.
(d)not related to the solubility of gases.
50% studentsanswered this correctly

Important Questions on Solutions
EASY
12th West Bengal Board
IMPORTANT
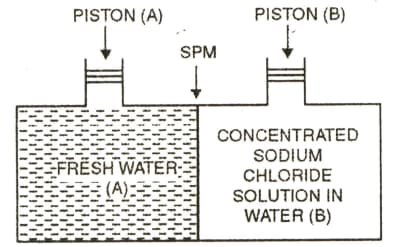
Consider the Fig. and make the correct option.
EASY
12th West Bengal Board
IMPORTANT
EASY
12th West Bengal Board
IMPORTANT
(A) In bromoethane and chloroethane mixture intermolecular interactions of and type are nearly same as type interactions.
(B) In ethanol and acetone mixture, or type intermolecular interactions are stronger than type interactions.
(C) In chloroform and acetone mixture, or type intermolecular interactions are weaker than type interactions.
EASY
12th West Bengal Board
IMPORTANT
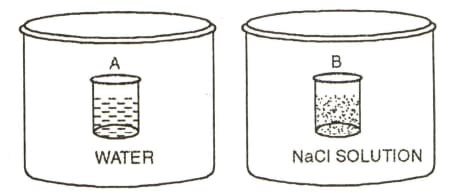
Two beakers of capacity were taken. One of these beakers, labelled as was filled with water whereas the beaker labelled was filled with of solution of At the same temperature, both the beakers were placed in closed containers of same material and same capacity as shown in Fig. At a given temperature, which of the following statements is correct about the vapour pressure of pure water and that of solution?
EASY
12th West Bengal Board
IMPORTANT
If two liquids and form minimum boiling azeotrope at some specific composition then
EASY
12th West Bengal Board
IMPORTANT
EASY
12th West Bengal Board
IMPORTANT
On the basis of information given below, mark the correct option.
Information: On adding acetone to methanol some of the hydrogen bonds between methanol molecules break.
EASY
12th West Bengal Board
IMPORTANT
Arrange these gases in the order of their increasing solubility.
