EASY
Earn 100
The value of Henry's law constant for some gases at 293 K is given below. Arrange the gases in the increasing order of their solubility.
He: 144.97 kbar, : 69.16 kbar,
: 76.48 kbar, : 34.86 kbar
(a)
(b)
(c)
(d)
50% studentsanswered this correctly
Important Questions on Solutions
EASY
EASY
MEDIUM

The gas with the highest value of Henry's law constant is
MEDIUM
Henry's constant (in kbar) for four gases and in water at is given below :
(density of water at ) This table implies that :
EASY
MEDIUM
MEDIUM
[Henry's law constant for at bar; density of water at ; mass of ; molar mass of water ]
MEDIUM
EASY
EASY
EASY
EASY
From the graph, the value of Henry's constant for the solubility of gas in cyclohexane is
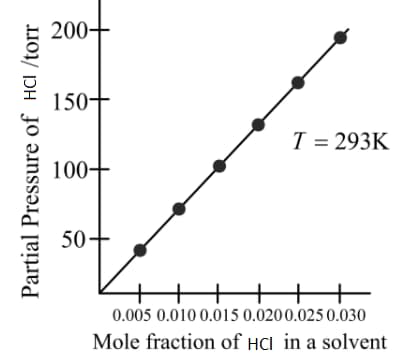
HARD
EASY
The oxygen dissolved in water exerts a partial pressure of in the vapour above water. The molar solubility of oxygen in water is ______
(Round off to the Nearest Integer).
[Given : Henry's law constant for Density of water with dissolved oxygen]
MEDIUM
MEDIUM
EASY
HARD

