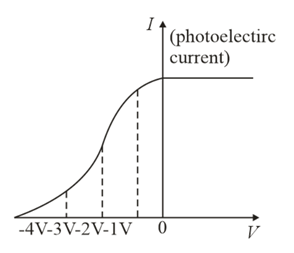
The value of stopping potential in the following diagram

Important Questions on Structure of Atom
The minimum energy that must be possessed by photons in order to produce the photoelectric effect with platinum metal is:
[Given: The threshold frequency of platinum is and ]
Given below are two statements : one is labelled as Assertion A and the other is labelled as Reason R :
Assertion A: In the photoelectric effect, the electrons are ejected from the metal surface as soon as the beam of light of frequency greater than threshold frequency strikes the surface.
Reason R : When the photon of any energy strikes an electron in the atom, transfer of energy from the photon to the electron takes place.
In the light of the above statements, choose the most appropriate answer from the options given below :
Given below are two statements, one is labelled as Assertion A and the other is labelled as Reason R.
Assertion A: Loss of electron from hydrogen atom results in nucleus of size.
Reason R: Proton always exists in combined form.
In the light of the above statements, choose the most appropriate answer from the options given below:
[Given : , and ]
With regard to photoelectric effect, identify the CORRECT statement among the following:
What is the work function of the metal if the light of wavelength generates photoelectrons of velocity from it?
(Mass of electron , velocity of light , Planck's constant , Charge of electron )
When light of wavelength falls on a metal of threshold energy , the de-Broglie wavelength of emitted electrons is ________ . (Round off to the Nearest Integer).
[Use :
Values of work function for a few metals are given below
| Metal | ||||||
The number of metals which will show photoelectric effect when light of wavelength falls on it is _____
Given:
[Given : , Mass of electron ], Give an answer to the nearest integer value.
If is the momentum of the fastest electron ejected from a metal surface after the irradiation of light having wavelength then for momentum of the photoelectron, the wavelength of the light should be:
(Assume kinetic energy of ejected photoelectron to be very high in comparison to work function)

