HARD
JEE Main/Advance
IMPORTANT
Earn 100
The vapour pressure of ideal solution of benzene and toluene is at then what would be correct statement about same solution at
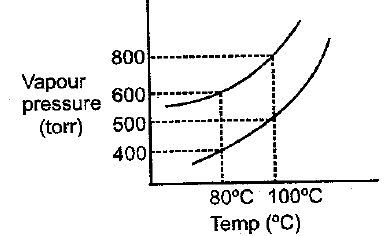

(a)Vapour pressure of solution torr.
(b)at torr pressure at no vapour form
(c)Composition of vapour is and
(d)Composition of liquid remain same at equilibrium condition at any temp.
50% studentsanswered this correctly

Important Questions on Solutions
MEDIUM
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
moles of and moles of are taken in separate beakers and enclosed in chamber Another moles of and moles of are mixed in a beaker and enclosed in chamber At equilibrium which of the following are not true. ( and are volatile liquids and they form ideal solution on mixing)
MEDIUM
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
Calculate molarity of final solution obtained by mixing I and II solution.
EASY
JEE Main/Advance
IMPORTANT
