EASY
NEET
IMPORTANT
Earn 100
The variation of graph with of a fixed mass of an ideal gas at constant temperature is graphically represented as shown in figure :
(a)
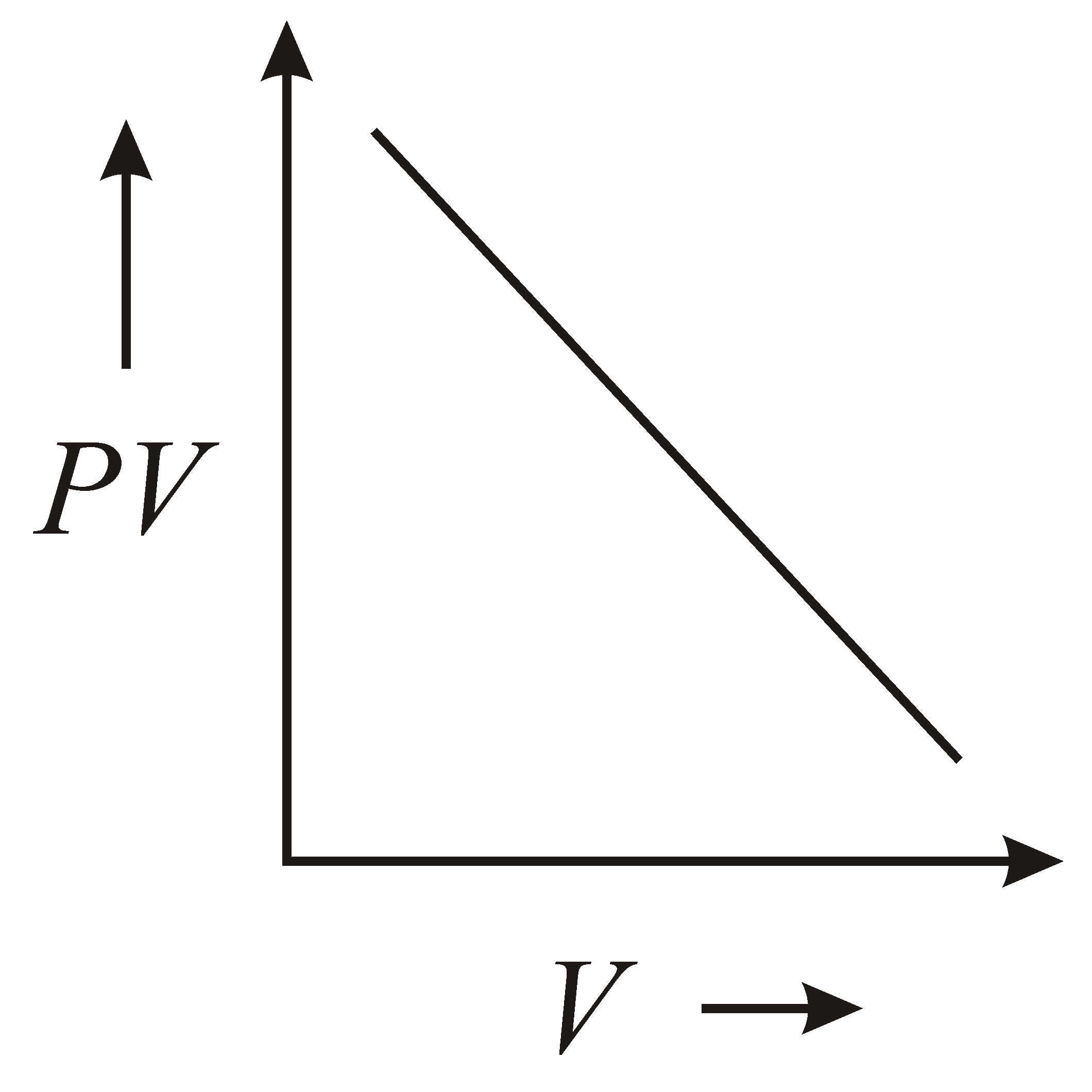
(b)
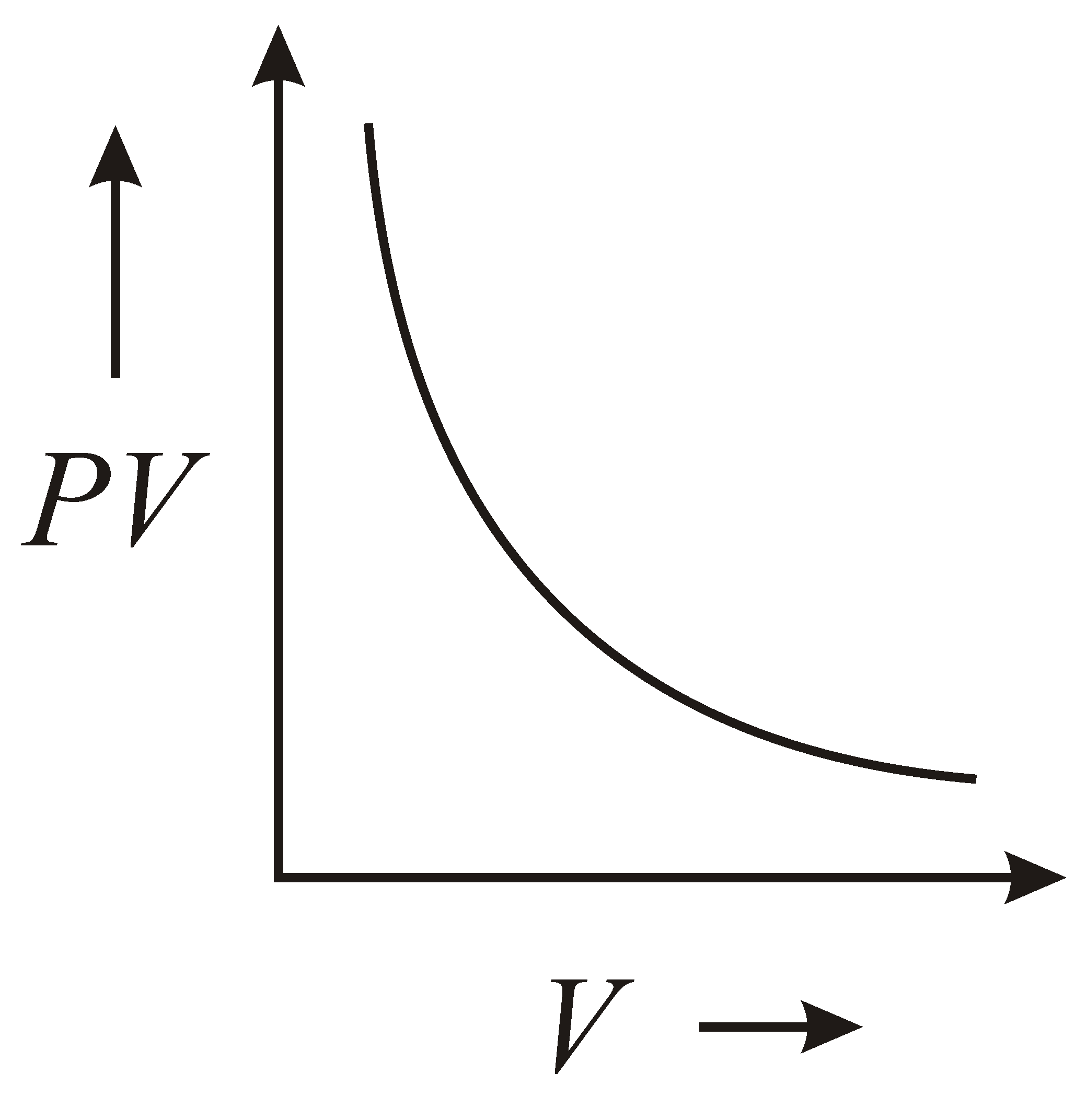
(c)
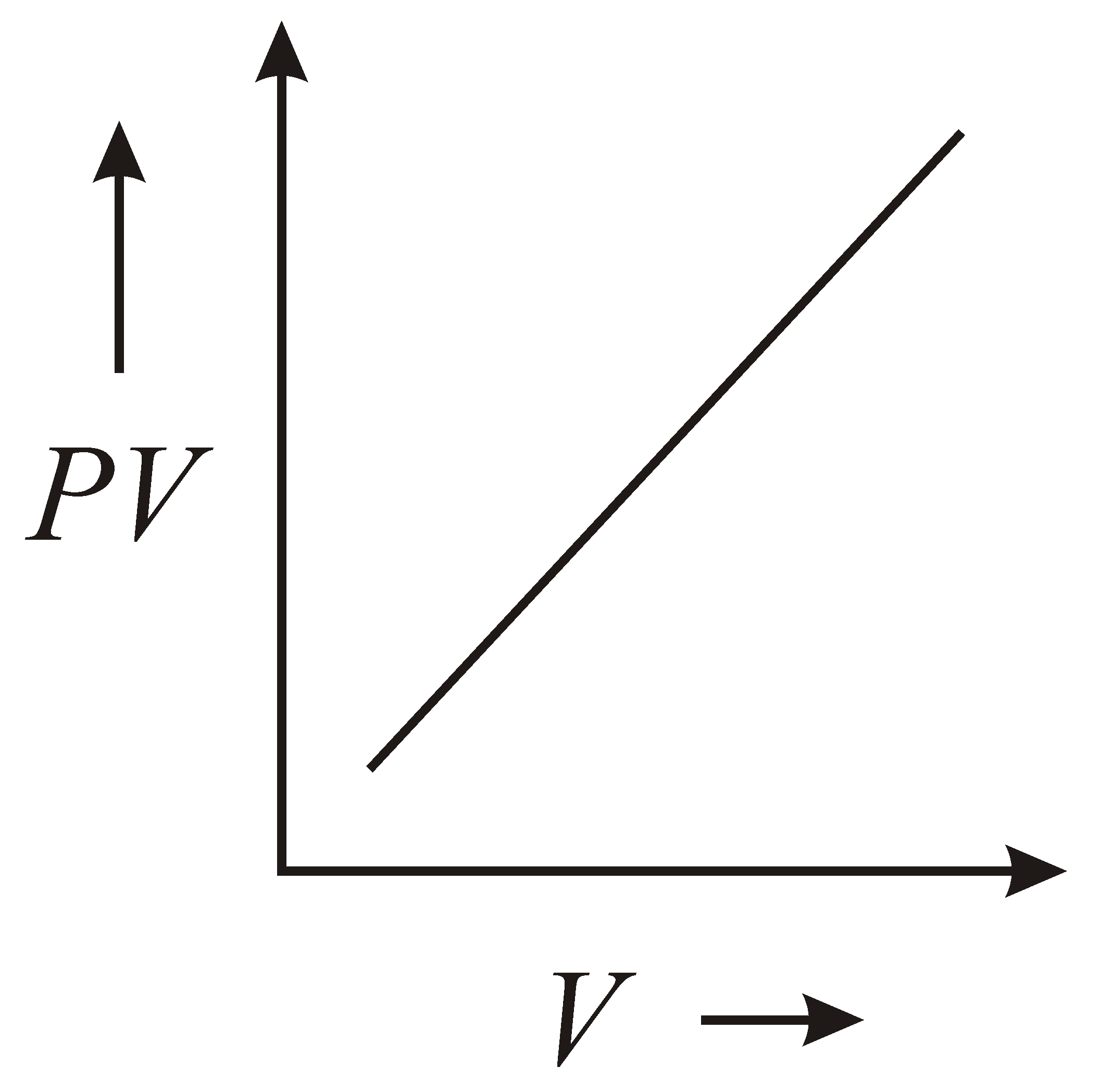
(d)
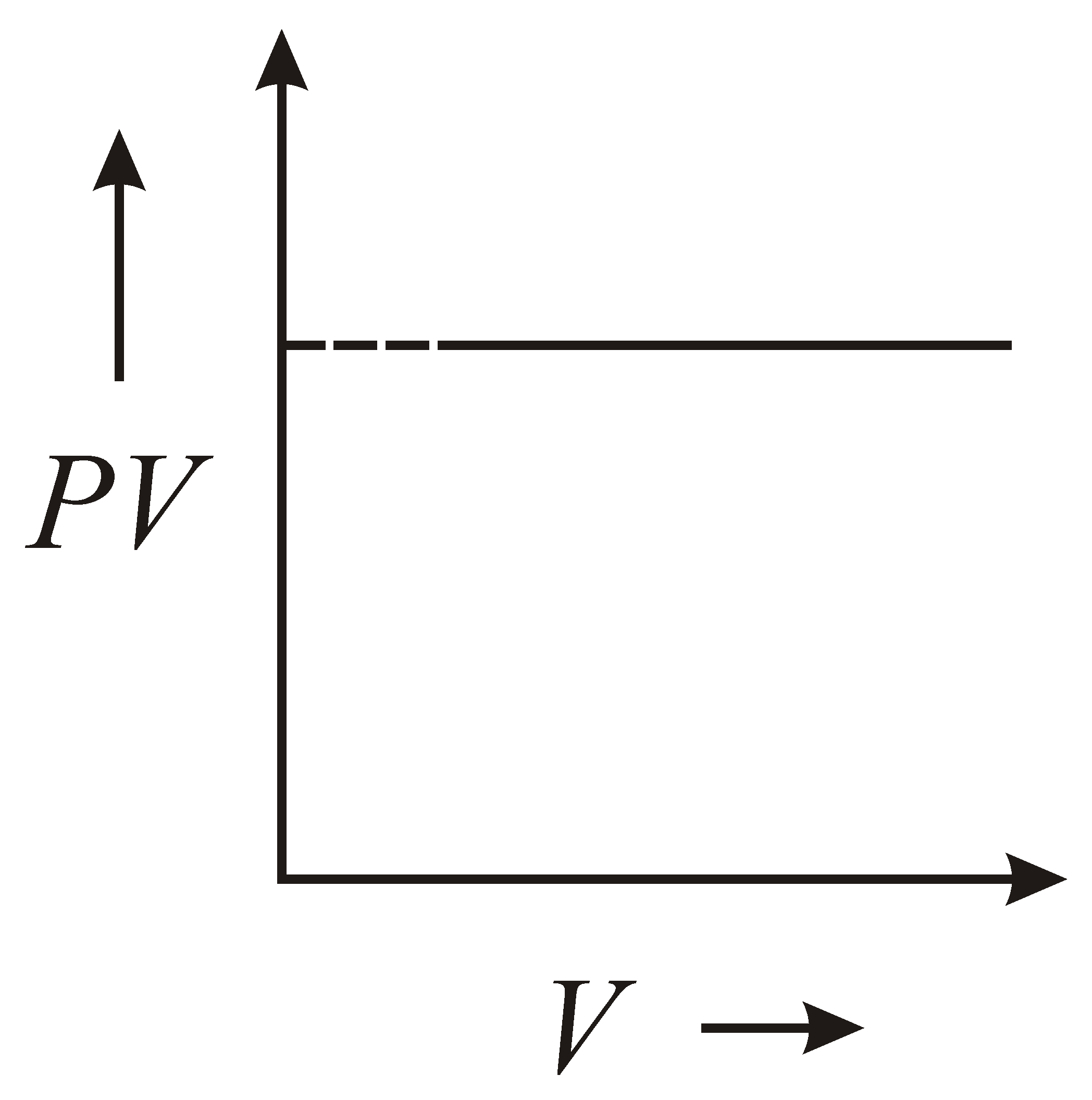
60% studentsanswered this correctly

Important Questions on Kinetic Theory of Gases
MEDIUM
NEET
IMPORTANT
The number of oxygen molecules in a cylinder of volume 1 m3 at a temperature of 27°C and pressure 13.8 Pa is :
(Boltzmann's constant k = 1.38 x 10–23 J K–1)
EASY
NEET
IMPORTANT
A cylinder contains 10 kg of gas at pressure of 107 N/m2. When final pressure is reduce to 2.5 x 106 N/m2 then quantiy of gas taken out of the cylinder will be : (temperature of gas is constant)
EASY
NEET
IMPORTANT
Hydrogen and helium gases of volume at same temperature and same pressure are mixed to have the same volume . The resulting pressure of the mixture will be:
EASY
NEET
IMPORTANT
The equation of state for 5g of oxygen at a pressure P and temperature T occupying a volume V, will be :—
(where R is the gas constant)
EASY
NEET
IMPORTANT
In kinetic theory of gases, it is assumed that molecular collisions are
EASY
NEET
IMPORTANT
The volume of an ideal gas is V at pressure P and temperature T. The mass of each molecule of the gas in m. The denisty of gas will be:-
(K is Boltzmann's constant)
EASY
NEET
IMPORTANT
The thermodynamic coordinates of a jar filled with gas are, and and another jar filled with another gas are , and , where the symbols have their usual meaning. The ratio of the number of molecules of jar to those jar is
EASY
NEET
IMPORTANT
At N.T.P. volume of a gas is changed to one fourth volume, at constant temperature then the new pressure will be :
