MEDIUM
Earn 100
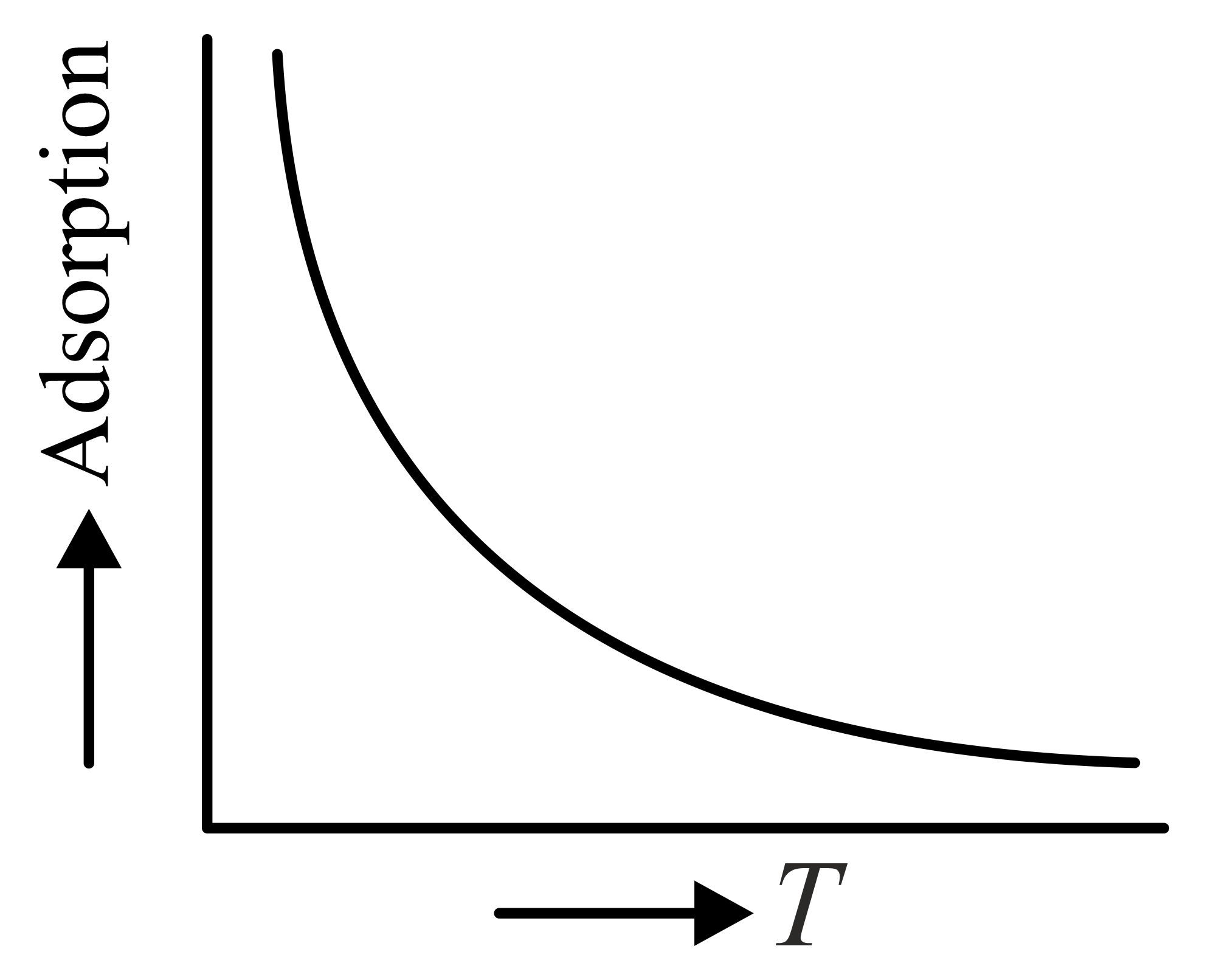
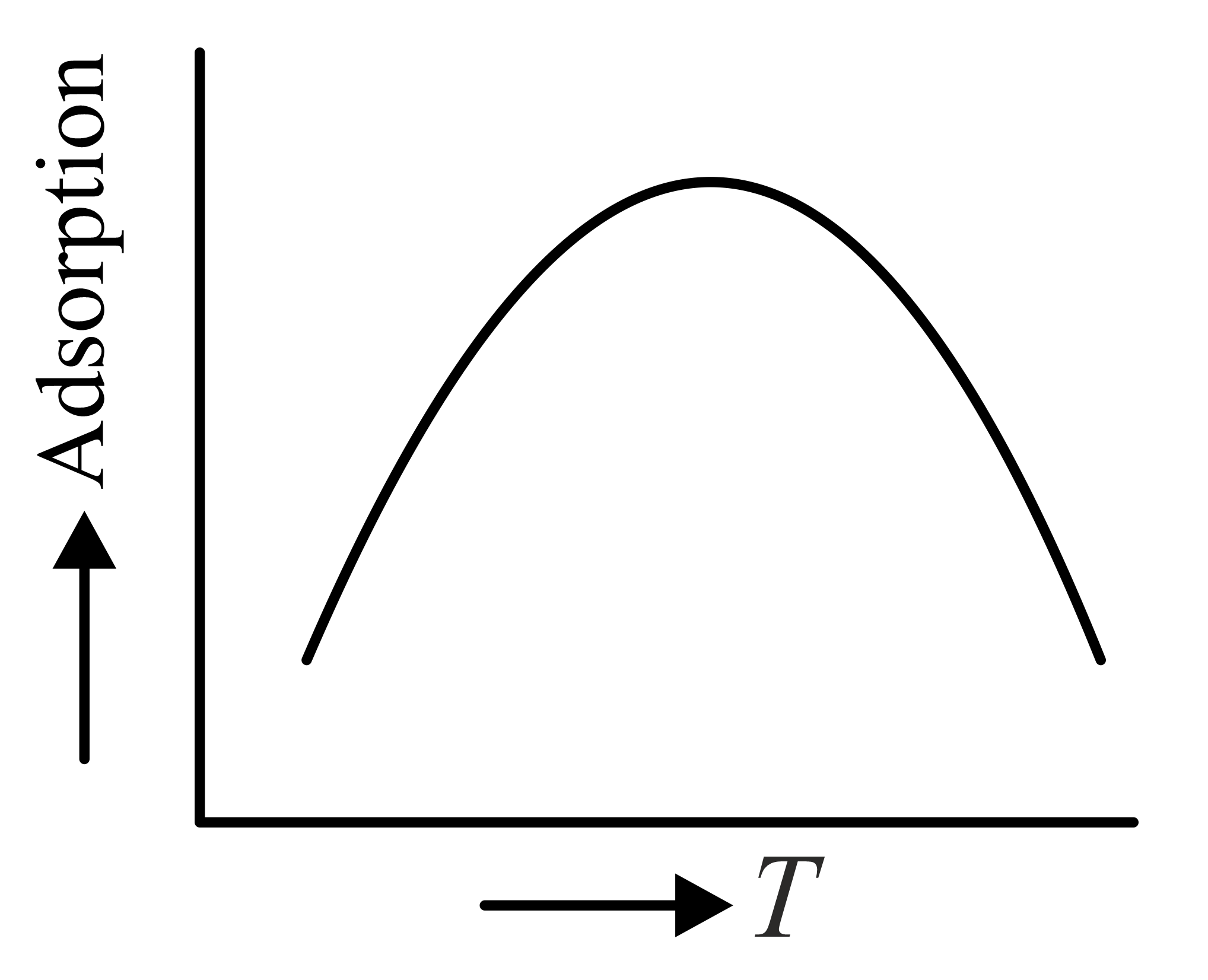
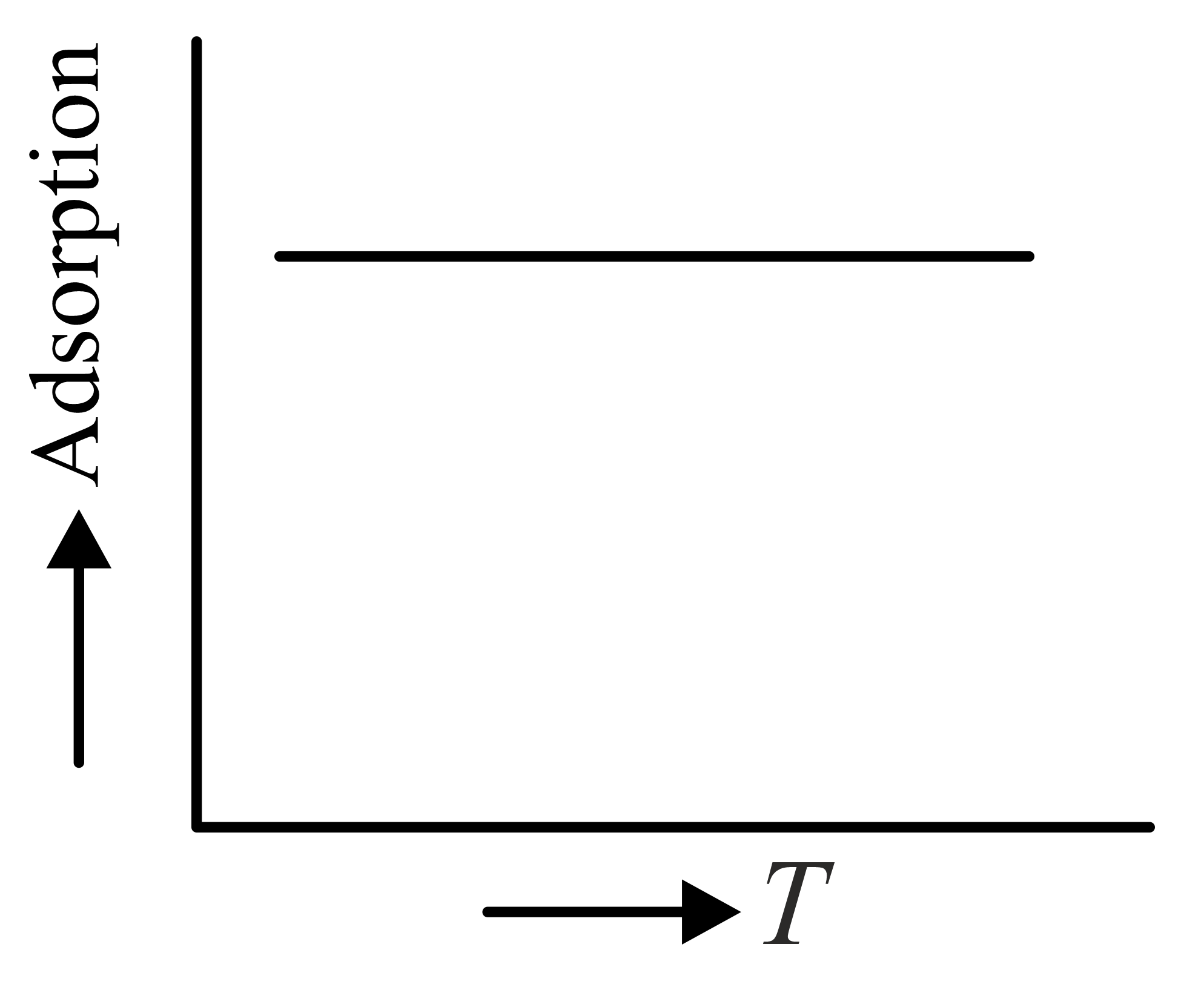
The variation of physical adsorption with temperature is shown by
(a)
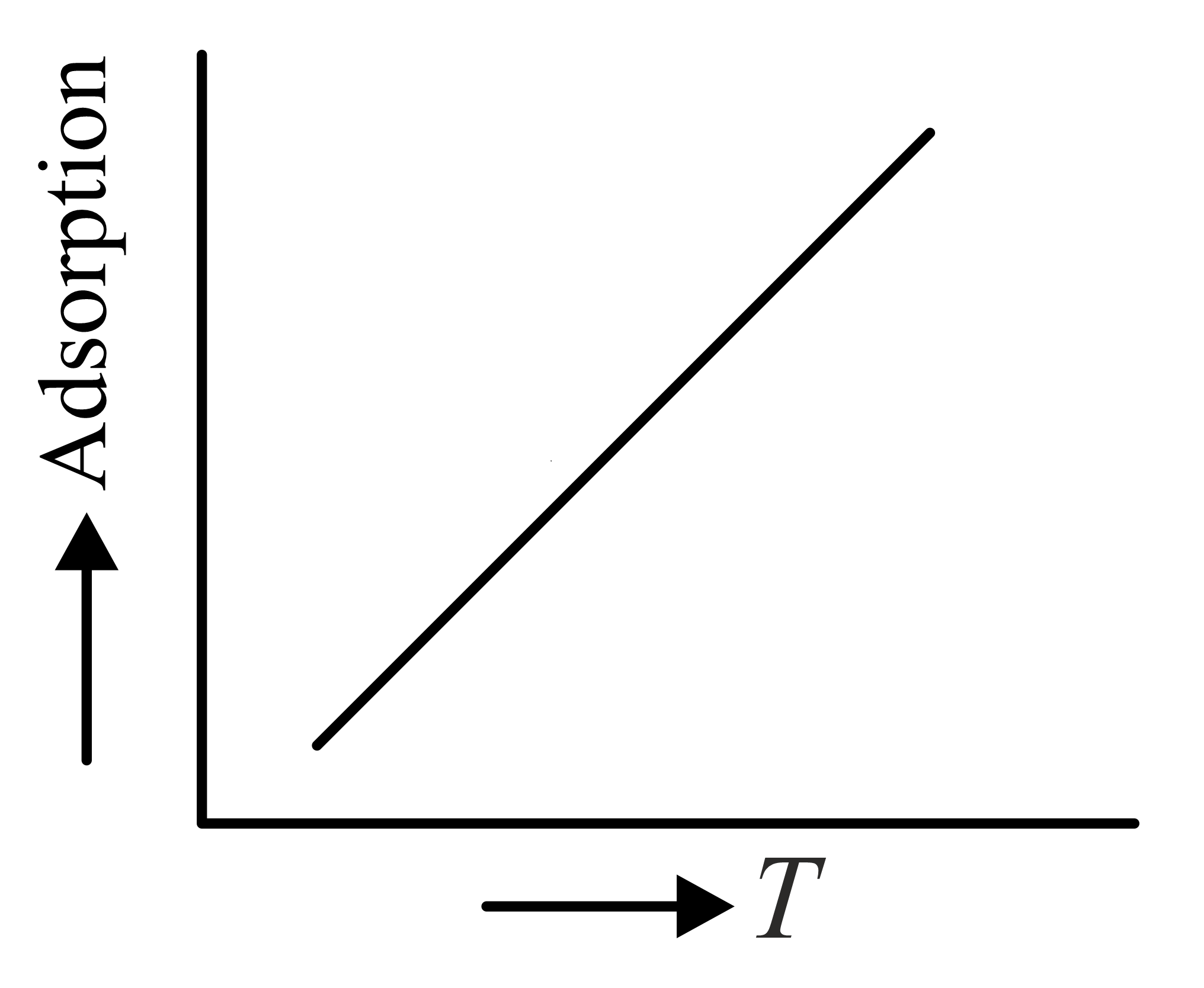
(b)
(c)
(d)
50% studentsanswered this correctly
Important Questions on Surface Chemistry
MEDIUM
MEDIUM
EASY
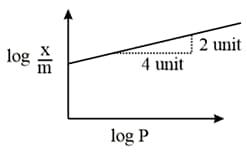
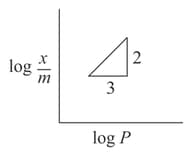
( is the mass of the gas adsorbed per gram of adsorbent)
MEDIUM
EASY
MEDIUM
MEDIUM
MEDIUM
(a) becomes less negative as adsorption proceeds.
(b) On a given adsorbent, ammonia is adsorbed more than nitrogen gas.
(c) On adsorption, the residual force acting along the surface of the adsorbent increases
(d) With the increase in temperature, the equilibrium concentration of adsorbate increases.
EASY
EASY
EASY
Adsorption of the gas increases with:
EASY
EASY
EASY
EASY
EASY
EASY
MEDIUM
Given:
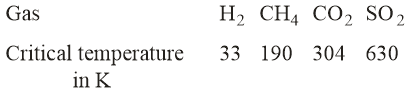
On the basis of data given above, predict which of the following gases shows the least adsorption on a definite amount of charcoal?
MEDIUM
Adsorption of a gas follows Freundlich adsorption isotherm. In the given plot, is the mass of the gas adsorbed on mass of the adsorbent at pressure is proportional to: