The work done when moles of an ideal gas expands reversibly and isothermally from a volume of to at is
Important Questions on Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.
[ Use ]
How much heat will be absorbed by mole of an ideal gas, if it is expanded reversibly from to at ?
One mole of an ideal gas at is taken from to as shown in the given indicator diagram. The work done by the system will be _______ [Given, ] (Round off to the nearest integer)
(Round off to the nearest integer)
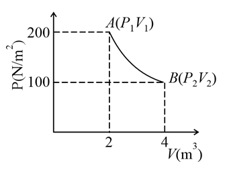
At of iron reacts with to form . The evolved hydrogen gas expands against a constant pressure of . The work done by the gas during this expansion is -_______ .
(Round off to the Nearest Integer)
[Given : . Assume, hydrogen is an ideal gas]
[Atomic mass off Fe is ]
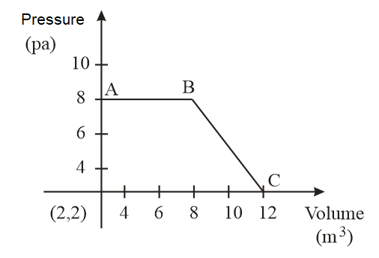
The magnitude of work done by a gas that undergoes a reversible expansion along the path shown in the figure is _________.
Under the isothermal condition, a gas at expands from to against a constant external pressure of bar. The work done by the gas is
(Given that bar)
(Round off to the Nearest Integer)
[Use :
[Assume volume of is much smaller than volume of . Assume treated as an ideal gas]
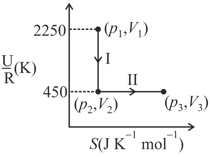
( : internal energy, entropy, pressure, volume, : gas constant)
(Given: molar heat capacity at constant volume, of the gas is )
Give your answer as the nearest integer.
Two identical samples of gas are allowed to expand isothermally and adiabatically. The amount of work done id then
The three processes in a thermodynamic cycle shown in the figure are : Process is isothermal; Process is isochoric (volume remains constant); Process is adiabatic.
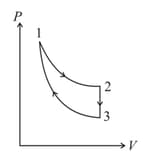
The total work done by the ideal gas in this cycle is, The internal energy decreases by, in the isochoric process. The work done by the gas in the adiabatic process is, . The heat added to the system in the isothermal process is

