EASY
9th Foundation
IMPORTANT
Earn 100
There are two atomic species and , such that
Atomic species
Protons
Neutrons
Which of the following statements is true about and ?
(a) and are isobars.
(b) and have different chemical properties.
(c) and have different physical properties.
(d)All are correct.
11.43% studentsanswered this correctly

Important Questions on Structure of Atom
EASY
9th Foundation
IMPORTANT
Match column I with column II and select the correct option from the given codes.
| Column I | Column II | ||
| (a) | Ions | (i) | Same mass number, different atomic numbers |
| (b) | Isobars | (ii) | Same number of neutrons, different atomic numbers, different mass numbers |
| (c) | Isotopes | (iii) | Formed by loss or gain of electrons |
| (d) | Isotones | (iv) | Combining capacity of an atom |
| (e) | Valency | (v) | Same atomic number, different mass numbers |
EASY
9th Foundation
IMPORTANT
Atomic number , Mass number
Atomic number , Mass number
Which of the following is correct about these two atoms?
EASY
9th Foundation
IMPORTANT
EASY
9th Foundation
IMPORTANT
EASY
9th Foundation
IMPORTANT
Which of the following are isobars?
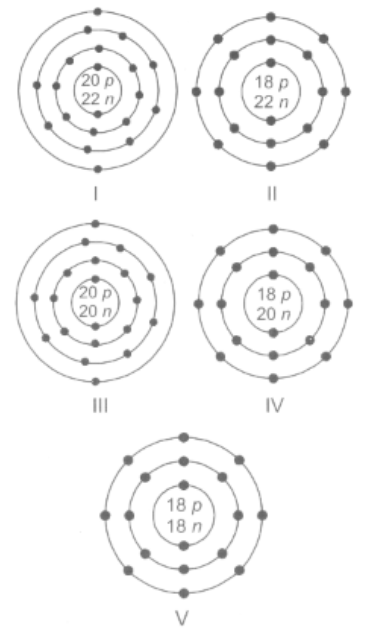
EASY
9th Foundation
IMPORTANT
Statement 1: Bohr's orbits are called stationary orbits.
Statement 2 : Electrons remain stationary in these orbits for some time.
EASY
9th Foundation
IMPORTANT
MEDIUM
9th Foundation
IMPORTANT
Which of the following statements are correct regarding the elements given below?
I The correct order of increasing proton number is
II The correct order of increasing mass number is
III There is difference in the orders of proton number and mass number.
IV The number of protons is equal to number of neutrons in all the given elements.
